
General, Organic, & Biological Chemistry
3rd Edition
ISBN: 9780073511245
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.1, Problem 13.3P
Complete the structure of zingiberene, a component of ginger root, by drawing in all H’s on the carbon skeleton. Then draw a skeletal structure for zingiberene.
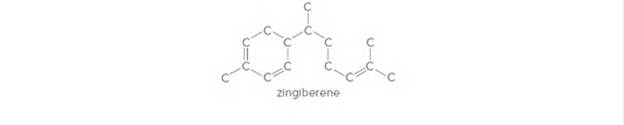
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Butane is an unbranched alkane with the condensed structure CH,CH,CH,CH,.
Draw the complete structure of butane. Show all hydrogen atoms.
Select
Draw
Rings
More
C
H
Draw the skeletal structure of butane in line-bond (line-angle) mode. Do not show hydrogen atoms.
Select
Draw
Rings
More
C
Draw and name all possible skeletal isomers of compounds having the molecular
formula C6H14
. All compounds are alkanes (carbon atoms are connected by single
bonds only).
See image below
Chapter 13 Solutions
General, Organic, & Biological Chemistry
Ch. 13.1 - Convert each condensed structure to a complete...Ch. 13.1 - Prob. 13.2PCh. 13.1 - Complete the structure of zingiberene, a component...Ch. 13.2 - Prob. 13.4PCh. 13.2 - Prob. 13.5PCh. 13.2 - Prob. 13.6PCh. 13.3 - Prob. 13.7PCh. 13.3 - Prob. 13.8PCh. 13.3 - Prob. 13.9PCh. 13.3 - Prob. 13.10P
Ch. 13.3 - Prob. 13.11PCh. 13.3 - Prob. 13.12PCh. 13.3 - Prob. 13.13PCh. 13.4 - Prob. 13.14PCh. 13.6 - Prob. 13.15PCh. 13.6 - Prob. 13.16PCh. 13.6 - Prob. 13.17PCh. 13.6 - Prob. 13.18PCh. 13.6 - Prob. 13.19PCh. 13.7 - Prob. 13.20PCh. 13.8 - Prob. 13.21PCh. 13.8 - Prob. 13.22PCh. 13.10 - Give the IUPAC name of each compound.
Ch. 13.10 - Prob. 13.24PCh. 13.11 - Prob. 13.25PCh. 13.12 - Prob. 13.26PCh. 13.13 - Prob. 13.27PCh. 13.13 - Prob. 13.28PCh. 13 - Anethole, the major constituent of anise oil, is...Ch. 13 - Prob. 13.30PCh. 13 - What is the molecular formula for a hydrocarbon...Ch. 13 - Prob. 13.32PCh. 13 - Prob. 13.33PCh. 13 - Prob. 13.34PCh. 13 - Prob. 13.35PCh. 13 - Prob. 13.36PCh. 13 - Give the IUPAC name for each molecule depicted in...Ch. 13 - Give the IUPAC name for each molecule depicted in...Ch. 13 - Give the IUPAC name for each compound. a....Ch. 13 - Give the IUPAC name for each compound. d....Ch. 13 - Prob. 13.41PCh. 13 - Prob. 13.42PCh. 13 - Give the structure corresponding to each IUPAC...Ch. 13 - Prob. 13.44PCh. 13 - Each of the following IUPAC names is incorrect....Ch. 13 - Each of the following IUPAC names is incorrect....Ch. 13 - Prob. 13.47PCh. 13 - Prob. 13.48PCh. 13 - Prob. 13.49PCh. 13 - Label the carbon-carbon double bond as cis or...Ch. 13 - Prob. 13.51PCh. 13 - Prob. 13.52PCh. 13 - Prob. 13.53PCh. 13 - Prob. 13.54PCh. 13 - Prob. 13.55PCh. 13 - Prob. 13.56PCh. 13 - Prob. 13.57PCh. 13 - Prob. 13.58PCh. 13 - Prob. 13.59PCh. 13 - Prob. 13.60PCh. 13 - What alkyd halide is formed when each alkene is...Ch. 13 - Prob. 13.62PCh. 13 - Prob. 13.63PCh. 13 - Prob. 13.64PCh. 13 - What alkene is needed as a starting material to...Ch. 13 - Prob. 13.66PCh. 13 - Prob. 13.67PCh. 13 - Prob. 13.68PCh. 13 - Prob. 13.69PCh. 13 - Prob. 13.70PCh. 13 - Prob. 13.71PCh. 13 - Prob. 13.72PCh. 13 - Prob. 13.73PCh. 13 - Prob. 13.74PCh. 13 - Prob. 13.75PCh. 13 - Prob. 13.76PCh. 13 - Prob. 13.77PCh. 13 - Prob. 13.78PCh. 13 - Prob. 13.79PCh. 13 - Prob. 13.80PCh. 13 - Prob. 13.81PCh. 13 - Are o-bromochlorobenzene and m-bromochlorobenzene...Ch. 13 - Give the structure corresponding to each IUPAC...Ch. 13 - Give the structure corresponding to each IUPAC...Ch. 13 - Prob. 13.85PCh. 13 - Prob. 13.86PCh. 13 - Prob. 13.87PCh. 13 - Prob. 13.88PCh. 13 - Prob. 13.89PCh. 13 - Prob. 13.90PCh. 13 - Prob. 13.91PCh. 13 - Prob. 13.92PCh. 13 - Prob. 13.93PCh. 13 - Eleostearic acid is an unsaturated fatty acid...Ch. 13 - Prob. 13.95PCh. 13 - Prob. 13.96PCh. 13 - Prob. 13.97PCh. 13 - Prob. 13.98PCh. 13 - Prob. 13.99PCh. 13 - Prob. 13.100PCh. 13 - Prob. 13.101PCh. 13 - Prob. 13.102PCh. 13 - Answer the following questions about compound A,...Ch. 13 - Prob. 13.104PCh. 13 - Prob. 13.105PCh. 13 - Prob. 13.106PCh. 13 - Prob. 13.107CPCh. 13 - Prob. 13.108CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw a skeletal ("line") structure of this molecule: CH3 CH3 C-CH2–0-CH3arrow_forwardDraw the structure of a hydrocarbon with molecular formula C6H10 that also contains: (a) a carbon–carbon triple bond; (b) two carbon–carbon double bonds; (c) one ring and one C=C.arrow_forward= ORGANIC CHEMISTRY Drawing a skeletal structure from a condensed structure Draw a skeletal ("line") structure of this molecule: CH3 i CH3 C-CH₂- -C-CH3 CH3 —arrow_forward
- Hexane is an unbranched alkane with the condensed structure CH, CH,CH,CH, CH, CH, . Draw the complete structure of hexane. Show all hydrogen atoms. Select Draw Rings More // Draw the skeletal structure of hexane in line-bond (or line-angle) mode. Do not show hydrogen atoms. Draw Rings More Select C.arrow_forwardFunctional group Organic compound Elements C=C C-OH C with 4 single bonds Closed loop of C's, alternative double bonds and single bonds and odd number of pi bonds C-CO -Carrow_forward4. Draw arrows on the given structure, draw the best resonance form, then draw the resonance hybrid (place lone pairs and charges in all structures where appropriate): 5. Convert the given condensed structure to (a) skeletal, and (b) Lewis structure. CH3OCH=C(CH3)CH=CHCO₂CH3arrow_forward
- There are 17 constitutional isomers with the molecular formula of C5H13N. Draw all 17 of these isomers as skeletal structures and give the SMILES for each.arrow_forwardDraw the condensed structure of an isomer of this molecule:arrow_forwardPredict the product of this organic reaction: CH3 O Pt CH3 CH C H + H2 P Specifically, in the drawing area below draw the condensed structure of P. Click anywhere to draw the first atom of your structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY