
General, Organic, & Biological Chemistry
3rd Edition
ISBN: 9780073511245
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Chapter 13, Problem 13.80P
Interpretation Introduction
(a)
Interpretation:
The name of the aromatic compound given below needs to be deduced:
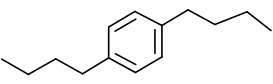
Concept Introduction:
Aromatic compounds are predominantly characterized by the presence of a benzene (C6H6)ring- The parent ring can be identified based on the principal
functional groups attached to the carbon atoms in the ring. These groups could be methyl (-CH3 which gives toluene), amino (-NH2 which forms aniline), hydroxyl (-OH which forms phenol) and so on. - If two or more substituents are present in the ring, the substituents are named in alphabetical order and the ring is numbered such that the first substituent gets the lowest number order.
Interpretation Introduction
(b)
Interpretation:
The name of the aromatic compound given below needs to be deduced:
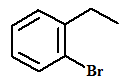
Concept Introduction:
- Aromatic compounds are predominantly characterized by the presence of a benzene (C6H6)ring
- The parent ring can be identified based on the principal functional groups attached to the carbon atoms in the ring. These groups could be methyl (-CH3 which gives toluene), amino (-NH2 which forms aniline), hydroxyl (-OH which forms phenol) and so on.
- If two or more substituents are present in the ring, the substituents are named in alphabetical order and the ring is numbered such that the first substituent gets the lowest number order.
Interpretation Introduction
(c)
Interpretation:
The name of the aromatic compound given below needs to be deduced:
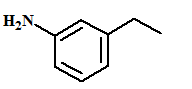
Concept Introduction:
- Aromatic compounds are predominantly characterized by the presence of a benzene (C6H6)ring
- The parent ring can be identified based on the principal functional groups attached to the carbon atoms in the ring. These groups could be methyl (-CH3 which gives toluene), amino (-NH2 which forms aniline), hydroxyl (-OH which forms phenol) and so on.
- If two or more substituents are present in the ring, the substituents are named in alphabetical order and the ring is numbered such that the first substituent gets the lowest number order.
Interpretation Introduction
(d)
Interpretation:
The name of the aromatic compound given below needs to be deduced
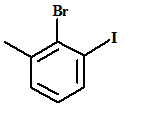
Concept Introduction:
- Aromatic compounds are predominantly characterized by the presence of a benzene (C6H6)ring
- The parent ring can be identified based on the principal functional groups attached to the carbon atoms in the ring. These groups could be methyl (-CH3 which gives toluene), amino (-NH2 which forms aniline), hydroxyl (-OH which forms phenol) and so on.
- If two or more substituents are present in the ring, the substituents are named in alphabetical order and the ring is numbered such that the first substituent gets the lowest number order.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?
Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃
Can I please get help with this?
Chapter 13 Solutions
General, Organic, & Biological Chemistry
Ch. 13.1 - Convert each condensed structure to a complete...Ch. 13.1 - Prob. 13.2PCh. 13.1 - Complete the structure of zingiberene, a component...Ch. 13.2 - Prob. 13.4PCh. 13.2 - Prob. 13.5PCh. 13.2 - Prob. 13.6PCh. 13.3 - Prob. 13.7PCh. 13.3 - Prob. 13.8PCh. 13.3 - Prob. 13.9PCh. 13.3 - Prob. 13.10P
Ch. 13.3 - Prob. 13.11PCh. 13.3 - Prob. 13.12PCh. 13.3 - Prob. 13.13PCh. 13.4 - Prob. 13.14PCh. 13.6 - Prob. 13.15PCh. 13.6 - Prob. 13.16PCh. 13.6 - Prob. 13.17PCh. 13.6 - Prob. 13.18PCh. 13.6 - Prob. 13.19PCh. 13.7 - Prob. 13.20PCh. 13.8 - Prob. 13.21PCh. 13.8 - Prob. 13.22PCh. 13.10 - Give the IUPAC name of each compound.
Ch. 13.10 - Prob. 13.24PCh. 13.11 - Prob. 13.25PCh. 13.12 - Prob. 13.26PCh. 13.13 - Prob. 13.27PCh. 13.13 - Prob. 13.28PCh. 13 - Anethole, the major constituent of anise oil, is...Ch. 13 - Prob. 13.30PCh. 13 - What is the molecular formula for a hydrocarbon...Ch. 13 - Prob. 13.32PCh. 13 - Prob. 13.33PCh. 13 - Prob. 13.34PCh. 13 - Prob. 13.35PCh. 13 - Prob. 13.36PCh. 13 - Give the IUPAC name for each molecule depicted in...Ch. 13 - Give the IUPAC name for each molecule depicted in...Ch. 13 - Give the IUPAC name for each compound. a....Ch. 13 - Give the IUPAC name for each compound. d....Ch. 13 - Prob. 13.41PCh. 13 - Prob. 13.42PCh. 13 - Give the structure corresponding to each IUPAC...Ch. 13 - Prob. 13.44PCh. 13 - Each of the following IUPAC names is incorrect....Ch. 13 - Each of the following IUPAC names is incorrect....Ch. 13 - Prob. 13.47PCh. 13 - Prob. 13.48PCh. 13 - Prob. 13.49PCh. 13 - Label the carbon-carbon double bond as cis or...Ch. 13 - Prob. 13.51PCh. 13 - Prob. 13.52PCh. 13 - Prob. 13.53PCh. 13 - Prob. 13.54PCh. 13 - Prob. 13.55PCh. 13 - Prob. 13.56PCh. 13 - Prob. 13.57PCh. 13 - Prob. 13.58PCh. 13 - Prob. 13.59PCh. 13 - Prob. 13.60PCh. 13 - What alkyd halide is formed when each alkene is...Ch. 13 - Prob. 13.62PCh. 13 - Prob. 13.63PCh. 13 - Prob. 13.64PCh. 13 - What alkene is needed as a starting material to...Ch. 13 - Prob. 13.66PCh. 13 - Prob. 13.67PCh. 13 - Prob. 13.68PCh. 13 - Prob. 13.69PCh. 13 - Prob. 13.70PCh. 13 - Prob. 13.71PCh. 13 - Prob. 13.72PCh. 13 - Prob. 13.73PCh. 13 - Prob. 13.74PCh. 13 - Prob. 13.75PCh. 13 - Prob. 13.76PCh. 13 - Prob. 13.77PCh. 13 - Prob. 13.78PCh. 13 - Prob. 13.79PCh. 13 - Prob. 13.80PCh. 13 - Prob. 13.81PCh. 13 - Are o-bromochlorobenzene and m-bromochlorobenzene...Ch. 13 - Give the structure corresponding to each IUPAC...Ch. 13 - Give the structure corresponding to each IUPAC...Ch. 13 - Prob. 13.85PCh. 13 - Prob. 13.86PCh. 13 - Prob. 13.87PCh. 13 - Prob. 13.88PCh. 13 - Prob. 13.89PCh. 13 - Prob. 13.90PCh. 13 - Prob. 13.91PCh. 13 - Prob. 13.92PCh. 13 - Prob. 13.93PCh. 13 - Eleostearic acid is an unsaturated fatty acid...Ch. 13 - Prob. 13.95PCh. 13 - Prob. 13.96PCh. 13 - Prob. 13.97PCh. 13 - Prob. 13.98PCh. 13 - Prob. 13.99PCh. 13 - Prob. 13.100PCh. 13 - Prob. 13.101PCh. 13 - Prob. 13.102PCh. 13 - Answer the following questions about compound A,...Ch. 13 - Prob. 13.104PCh. 13 - Prob. 13.105PCh. 13 - Prob. 13.106PCh. 13 - Prob. 13.107CPCh. 13 - Prob. 13.108CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can I please get help with this?arrow_forwardUse the Henderson-Hasselbalch equation to calculate pH of a buffer containing 0.050M benzoic acidand 0.150M sodium benzoate. The Ka of benzoic acid is 6.5 x 10-5arrow_forwardA. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forward
- What is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY