
Concept explainers
(a)
Interpretation:
The product formed should be determined on the reaction of following

Concept Introduction:
A
In a chemical reaction, the substance which is involved in conversion is said to be reactant, whereas, the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
Hydration reaction is an addition reaction in which the hydrogen and hydroxyl group (-OH) are bonded on un-statured carbon atoms of alkene to form alcohol.
Answer to Problem 13.62P

Explanation of Solution
To get the hydration product of any alkene, three steps must be followed:
- Locate the position of C=C in the molecule.
- Break the H-OH bond of the reagent.
- Add the -OH group atom to double-bonded C atom to form new C−OH single bonds in the molecule.
- Add one H atom to another double-bonded C atom to form new C−H single bonds in the molecule.
- The reaction follows the Markovnikov rule which states that the H atom of H-OH will bond to that double-bonded C atom which has more number of H atoms.
Hence, the hydration product is:
. 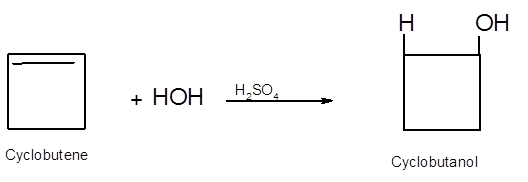
(b)
Interpretation:
The product formed should be determined on the reaction of following alkene with
(CH3)3 -C =C -(CH3)2
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant, whereas, the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
Hydration reaction is an addition reaction in which the hydrogen and hydroxyl group (-OH) are bonded on un-statured carbon atoms of alkene to form alcohol.
Answer to Problem 13.62P
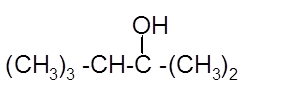
Explanation of Solution
To get the hydration product of any alkene, three steps must be followed;
- Locate the position of C=C in the molecule.
- Break the H-OH bond of the reagent.
- Add the -OH group atom to double-bonded C atom to form new C−OH single bonds in the molecule.
- Add one H atom to another double-bonded C atom to form new C−H single bonds in the molecule.
- The reaction follows the Markovnikov rule which states that the H atom of H-OH will bond to that double-bonded C atom which has more number of H atoms.
Hence, the hydration product is:
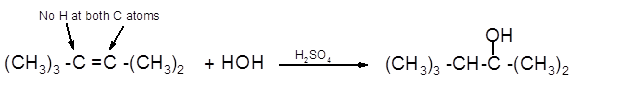
(c)
Interpretation:
The product formed should be determined on the reaction of following alkene with
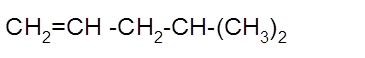
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant, whereas, the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
Hydration reaction is an addition reaction in which the hydrogen and hydroxyl group (-OH) are bonded on un-statured carbon atoms of alkene to form alcohol.
Answer to Problem 13.62P
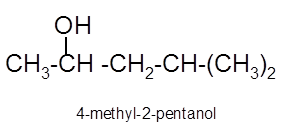
Explanation of Solution
To get the hydration product of any alkene, three steps must be followed;
- Locate the position of C=C in the molecule.
- Break the H-OH bond of the reagent.
- Add the -OH group atom to double-bonded C atom to form new C−OH single bonds in the molecule.
- Add one H atom to another double-bonded C atom to form new C−H single bonds in the molecule.
- The reaction follows the Markovnikov rule which states that the H atom of H-OH will bond to that double-bonded C atom which has more number of H atoms.
Hence, the hydration product is:
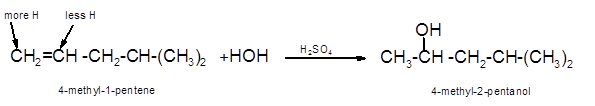
(d)
Interpretation:
The product formed should be determined on the reaction of following alkene with
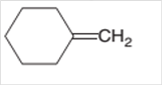
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant, whereas, the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
Hydration reaction is an addition reaction in which the hydrogen and hydroxyl group (-OH) are bonded on un-statured carbon atoms of alkene to form alcohol.
Answer to Problem 13.62P
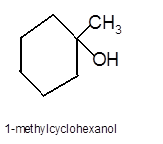
Explanation of Solution
To get the hydration product of any alkene, three steps must be followed;
- Locate the position of C=C in the molecule.
- Break the H-OH bond of the reagent.
- Add the -OH group atom to double-bonded C atom to form new C−OH single bonds in the molecule.
- Add one H atom to another double-bonded C atom to form new C−H single bonds in the molecule.
- The reaction follows the Markovnikov rule which states that the H atom of H-OH will bond to that double-bonded C atom which has more number of H atoms.
Hence the hydration product is:
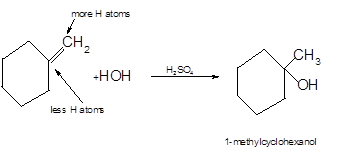
Want to see more full solutions like this?
Chapter 13 Solutions
General, Organic, & Biological Chemistry
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





