
Concept explainers
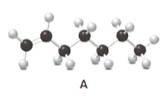
Answer the following questions about compound A, represent in the given ball-and-stick model.
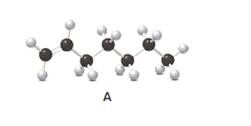
- Convert A to a condensed structure and give its IUPAC name.
- What product is formed when A is treated with H2 in the presence of metal catalyst?
- What product is formed when A is treated with H2O in the presence of H2SO4?
- What
polymer is formed when A ispolymerized ?
(a)
Interpretation:
Structure of the molecule A should be converted to a condensed structure and IUPAC name of the molecule should be given.
 = (
= (
Concept Introduction:
Alkenes are hydrocarbon molecules that consist of a carbon-carbon double bond which has the general formula of
In alkene nomenclature, the longest C chain will be considered as the parent/ main C chain. At the end of the alkane suffix -ene is added. The longest chain which includes the carbon-carbon double bond will be considered as the main carbon chain. The numbering of the main C chain is starting from the first substituent by giving the lower number to that substituent and as well as the double bond should get the lower number. All the substituents should be numbered and named. Two or more identical substituents should be indicated by using appropriate prefixes as di-. Names of the substituents should be alphabetized. Each substituent should have a position number which is mentioned in the main C chain.
Answer to Problem 13.103P
Condensed structure −
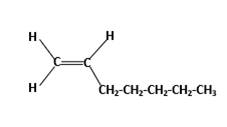
IUPAC name −Heptene
Explanation of Solution
In alkenes, there is a carbon-carbon double bond and different groups are connected to the two carbons. In most of the alkenes; out of four binding positions of carbon-carbon double bond, two of them are bind with two hydrogen atoms and the other two binding positions bind with two different alkyl groups. In this molecule, carbon atoms are represented in black and hydrogen atoms are represented in white color. The structure of a chemical compound is the arrangement of atoms and bonds in the space.
Condensed structure −
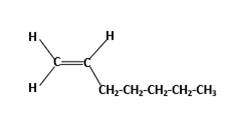
In this molecule, the main carbon chain which contains the carbon-carbon double bond is a seven-membered chain and there are no substituents in this molecule. According to the numbering of the main chain double bond gets the lowest number and it is number 1. Hence, the IUPAC name of the molecule is heptene.
(b)
Interpretation:
The resulting product should be identified after reacting
Concept Introduction:
Alkenes are hydrocarbon molecules that contain a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 13.103P
Explanation of Solution
Unsaturated (
(c)
Interpretation:
The resulting product should be identified after reacting
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Hydration reaction of alkenes follows the Markovnikov's rule.
Answer to Problem 13.103P
Explanation of Solution
Unsaturated (
Refer to the below reaction;
(d)
Interpretation:
The resulting polymer should be identified after polymerization of
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond inside a molecule.
Polymers are high molecular weight macromolecules which formed from covalently bonded monomer molecules.
Answer to Problem 13.103P
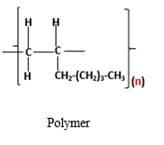
Explanation of Solution
Polymers are high molecular weight macromolecules which formed from covalently bonded monomer molecules. When forming polymers from alkene monomers, the weak bond which is in the carbon-carbon double bond is broken and new strong bond will form to connect the monomer molecules together. Hence; weak bond of the carbon-carbon double bond of

Want to see more full solutions like this?
Chapter 13 Solutions
General, Organic, & Biological Chemistry
- 10- 4000 20 20 30- %Reflectance 60 50- 09 60- 40- Date: Thu Feb 06 17:30:02 2025 (GMT-05:0(UnknownP Scans: 8 Resolution: 2.000 70 70 88 80 3500 3000 2500 90 100 00 Wavenumbers (cm-1) 2000 1500 2983.10 2359.13 1602.52 1584.22 1451.19 1391.87 1367.07 1314.37 1174.34 1070.13 1027.33 1714.16 1269.47 1000 1106.08 1001.14 937.02 873.60 850.20 780.22 686.91 674.38 643.09 617.98 02/06/25 16:38:20arrow_forwardd. Draw arrow-pushing mechanism for an enzymatic retro-aldol reaction of the following hexose. Use B: and/or HA as needed. OH OH سية HO OH OHarrow_forward4. Calculate the wavelength of a photon needed to excite a transition between neighbouring energy levels of a harmonic oscillator of effective mass equal to that of an oxygen atom and with a force constant of 544 N m¹.arrow_forward
- 2. Identify the strongest type of intermolecular force that exists between each pair of compounds: a. Ammonium chloride / H₂O b. OH C. d.arrow_forwardREPORT FOR EXPERIMENT 9 (continued) NAME F. Solubility vs. Temperature; Saturated and Unsaturated Solutions Data Table: Circle the choices which best describe your observations. NaCl 1.0 g +5 mL water 1.0 g +5 mL water +1.4 g dissolved completely? yes/no saturated or unsaturated? dissolved completely? yes/no saturated or unsaturated? 2.4 g +5 mL water +heat dissolved completely? yes/no saturated or unsaturated? 2.4 g +5 mL water after cooling dissolved completely? yes/no saturated or unsaturated? NHC dissolved completely? yes/no saturated or unsaturated? dissolved completely? yes/no saturated or unsaturated? dissolved completely? yes/no saturated or unsaturated? dissolved completely? yes/no saturated or unsaturated? G. Ionic Reactions in Solution 1. Write the word and formula equations representing the chemical reaction that occurred between the barium chloride solution, BaCl,(aq), and the sodium sulfate solution, Na SO (aq). Word Equation: Formula Equation: 2. (a) Which of the…arrow_forwardIn the drawing areas below, draw the two most expected stable conformations of the following molecule: ייון Be sure your drawings make it possible to distinguish between the conformations. After you've drawn the conformations, answer the question below the drawing areas. Х S : ☐ ☑ 5arrow_forward
- Add curved arrows to show the forming and breaking of bonds in the reaction below. :Br: H 2 Add/Remove step ☑ H-Br: G હે Parrow_forwardPlease correct answer and don't use hand ratingarrow_forwardSafari File Edit View History Bookmarks Window Help く < mylabmastering.pearson.com Wed Feb 12 8:44 PM ✩ + Apple Q Bing Google SignOutOptions M Question 36 - Lab HW BI... P Pearson MyLab and Mast... P Course Home Error | bartleby b Answered: If the biosynth... Draw a free-radical mechanism for the following reaction, forming the major monobromination product: ScreenPal - 2022 CHEM2... Access Pearson 2 CH3 Br-Br CH H3 Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron- flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. Include all free radicals by right-clicking on an atom on the canvas and then using the Atom properties to select the monovalent radical. ▸ View Available Hint(s) 0 2 DE [1] H EXP. CONT. H. Br-Br H FEB 12arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


