
Concept explainers
a.
Interpretation:
For the below compound the chirality centers has to be labeledand number of chiral centers should be determined.
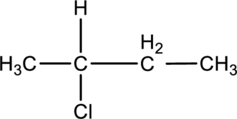
Concept introduction:
Chirality center:
When a carbon atom is bonded to four different groups, then that carbon is named as a chiral carbon and it is called as chirality center. Generally, the carbon atom contains tetrahedral valency, therefore it bonded to four atoms or molecules. The chiral center is bonded with four different elements or groups. If the single carbon atom is bonded to two or more identical group or atom, then it is also not considered as chiral carbon. In addition to this, if the carbon atom contains multiple bond then it does not able to bond with four different groups then itis not consideredto be as a chirality center.
b.
Interpretation:
For the below compound the chirality centers has to be labeled and number of chiral centers should be determined.
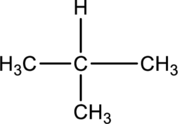
Concept introduction:
Refer to part “a”.
c.
Interpretation:
For the below compound the chirality centers has to be labeled and number of chiral centers should be determined.
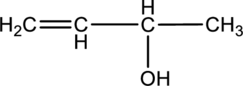
Concept introduction:
Refer to part “a.”.
d.
Interpretation:
For the below compound the chirality centers has to be labeled and number of chiral centers should be determined.
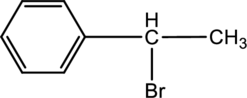
Concept introduction:
Refer to part ‘a.’.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
- predict the product formed by the reaction of one mole each of cyclohex-2-en-1-one and lithium diethylcuprate. Assume a hydrolysis step follows the additionarrow_forwardPlease handwriting for questions 1 and 3arrow_forwardIs (CH3)3NHBr an acidic or basic salt? What happens when dissolved in aqueous solution? Doesn't it lose a Br-? Does it interact with the water? Please advise.arrow_forward
- © Macmilla Finish resonance structure 3 Select Draw Templates More C H N 0 H H S Erase Which structure is the most stable (lowest energy) resonance contributor? The structure with the positive charge on nitrogen and negative charges on oxygen and sulfur. All structures are equal in stability. The structure with the positive charge on nitrogen and negative charges on sulfur and carbon. The structure with the positive charge on nitrogen and negative charges on oxygen and carbon. Q2Qarrow_forwardThree pure compounds are formed when 1.00 g samples of element x combine with, respectively, 0.472 g, 0.630 g, and 0.789 g of element z. The first compound has the formula x2Z3. find the empricial formula of the other two compoundsarrow_forwardDraw the product and the mechanism A. excess H*; 人 OH H*; B. C. D. excess OH ✓ OH H*; H₂O 1. LDA 2. H*arrow_forward
- In reactions whose kinetic equation is v = k[A]m, the rate coefficient k is always positive. Is this correct?arrow_forwardIf the concentration of A decreases exponentially with time, what is the rate equation? (A). -d[A] (B). dt d[A] = k[A] e-kt dtarrow_forwardGiven the first-order reaction: aA → products. State its kinetic equation.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

