
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.62AP
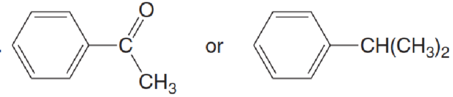
Which compound in each pair is more water soluble?
- a. CH3(C H2)6CHO or CH3(CH2)7OH
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What alkenes are formed when each alcohol is dehydrated with TSOH? Label the major product when a mixture results.
OH
Xom
OH
a.
OH
b.
CH₂CH3
OH
C.
d. CH₂CH₂CH₂CH₂OH
e.
Draw the products formed when each compound is treated with CH;CH,COCI, AICI3.
CH(CH3)2
N(CH)2
Br
CH3
CH(CH)2
a.
b.
C.
d.
е.
3. What product is formed when HO(CH₂) CHO is treated with each reagent?
c. Ag₂O, NH OH
d. Na₂Cr₂O₂, H₂SO4, H₂O
a. NaBH, CH₂OH
b. PCC
400
Chapter 12 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 12.1 - a. Label the hydroxyl groups, thiols, halogens,...Ch. 12.1 - Draw out each compound to clearly show what groups...Ch. 12.2 - Classify each alcohol as 1, 2, or 3.Ch. 12.2 - Classify each hydroxyl group in sorbitol as 1, 2,...Ch. 12.2 - Which compound in each pair has the higher boiling...Ch. 12.2 - Label each compound as water soluble or water...Ch. 12.2 - Give the IUPAC name for each compound.Ch. 12.2 - Give the structure corresponding to each name. a....Ch. 12.3 - Name each ether. a. CH3OCH2CH2CH2CH3 b....Ch. 12.3 - Prob. 12.10P
Ch. 12.3 - Which compound in each pair has the higher boiling...Ch. 12.5 - Prob. 12.12PCh. 12.5 - Prob. 12.13PCh. 12.6 - Prob. 12.14PCh. 12.6 - Prob. 12.15PCh. 12.6 - Give the structure corresponding to each name. a....Ch. 12.7 - Prob. 12.17PCh. 12.8 - Give the IUPAC name for each aldehyde. a....Ch. 12.8 - Prob. 12.19PCh. 12.8 - Give the IUPAC name for each aldehyde depicted in...Ch. 12.8 - Prob. 12.21PCh. 12.8 - Prob. 12.22PCh. 12.8 - Acetone and progesterone are two ketones that...Ch. 12.9 - Prob. 12.24PCh. 12.10 - Prob. 12.25PCh. 12.11 - Prob. 12.26PCh. 12.11 - Prob. 12.27PCh. 12.11 - Prob. 12.28PCh. 12.11 - Prob. 12.29PCh. 12.11 - Prob. 12.30PCh. 12.11 - Prob. 12.31PCh. 12.11 - Prob. 12.32PCh. 12 - Prob. 12.33UKCCh. 12 - Prob. 12.34UKCCh. 12 - Consider the following ball-and-stick model of an...Ch. 12 - Consider the following ball-and-stick model. a....Ch. 12 - Name each compound. a. CH3CH2OCH2CH2CH2CH3Ch. 12 - Name each compound. a. CH3OCH2CH2CH3 b....Ch. 12 - Answer the following questions about alcohol A. a....Ch. 12 - Answer the following questions about alcohol B. a....Ch. 12 - Prob. 12.41UKCCh. 12 - Prob. 12.42UKCCh. 12 - Prob. 12.43UKCCh. 12 - Prob. 12.44UKCCh. 12 - Prob. 12.45APCh. 12 - Prob. 12.46APCh. 12 - Prob. 12.47APCh. 12 - Prob. 12.48APCh. 12 - Prob. 12.49APCh. 12 - Prob. 12.50APCh. 12 - Prob. 12.51APCh. 12 - Prob. 12.52APCh. 12 - Prob. 12.53APCh. 12 - Give the structure corresponding to each name. a....Ch. 12 - Prob. 12.55APCh. 12 - Draw structures for the four constitutional...Ch. 12 - Prob. 12.57APCh. 12 - Rank the following compounds in order of...Ch. 12 - Explain why two four-carbon organic molecules have...Ch. 12 - Explain why the boiling point of CH3CH2CH2CH2OH...Ch. 12 - Which compound in each pair has the higher boiling...Ch. 12 - Which compound in each pair is more water soluble?...Ch. 12 - Prob. 12.63APCh. 12 - Prob. 12.64APCh. 12 - Prob. 12.65APCh. 12 - Prob. 12.66APCh. 12 - Prob. 12.67APCh. 12 - Xylitol is a nontoxic compound as sweet as table...Ch. 12 - Prob. 12.69APCh. 12 - Prob. 12.70APCh. 12 - Prob. 12.71APCh. 12 - Prob. 12.72APCh. 12 - Prob. 12.73APCh. 12 - Prob. 12.74APCh. 12 - Prob. 12.75APCh. 12 - Prob. 12.76APCh. 12 - Prob. 12.77APCh. 12 - Draw the structure corresponding to each name. a....Ch. 12 - Prob. 12.79APCh. 12 - Prob. 12.80APCh. 12 - What product is formed when each compound is...Ch. 12 - Prob. 12.82APCh. 12 - Prob. 12.83APCh. 12 - Prob. 12.84APCh. 12 - Prob. 12.85APCh. 12 - Prob. 12.86APCh. 12 - Prob. 12.87APCh. 12 - Label each of the following objects as chiral or...Ch. 12 - Prob. 12.89APCh. 12 - Prob. 12.90APCh. 12 - Prob. 12.91APCh. 12 - Prob. 12.92APCh. 12 - Prob. 12.93APCh. 12 - Prob. 12.94APCh. 12 - Prob. 12.95APCh. 12 - Prob. 12.96APCh. 12 - Prob. 12.97APCh. 12 - How are the compounds in each pair related? Are...Ch. 12 - Prob. 12.99APCh. 12 - Prob. 12.100APCh. 12 - Prob. 12.101APCh. 12 - Prob. 12.102APCh. 12 - Prob. 12.103APCh. 12 - Lactic acid [CH3CH(OH)CO2H] gives sour milk its...Ch. 12 - Prob. 12.105APCh. 12 - Prob. 12.106APCh. 12 - Prob. 12.107CPCh. 12 - Prob. 12.108CPCh. 12 - Prob. 12.109BTCCh. 12 - Prob. 12.110BTC
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the structure of compound A? 010 Compound A tot 01 Oll O III O IV OV || s ||| Compound A IV COOHarrow_forwardRank the compounds in each group in order of increasing water solubility. a. (CH3),CH, CH3OCH2CH3, CH3CH;CH,CH3, CH;CH,CH2OH b. Brarrow_forwardDraw the product formed when each compound is treated with two equivalents of CH;CH,CH,CHgMgBr followed by H,0. OCH3 a. b. C. CH;CH2 OCH2CH3arrow_forward
- Draw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. d. (CH3CH2)2CHCOCl, AlCl3 j. product in (d), then NH2NH2, – OHarrow_forwardExplain why HCl reacts more rapidly with compound A than compound B. A H H₂C H CH H Barrow_forwardDraw the products formed when each alcohol is oxidized with K 2Cr 2O 7. In some cases, no reaction occurs.arrow_forward
- Draw the products formed when phenol (C6H5OH) is treated with each set of reagents. a. [1] HNO3, H2SO4; [2] Sn, HCl b. [1] (CH3CH2)2CHCOCl, AlCl3; [2] Zn(Hg), HCl c. [1] CH3CH2Cl, AlCl3; [2] Br2, hν d. [1] (CH3)2CHCl, AlCl3; [2] KMnO4arrow_forwardIdentify each compound as an ether, hemiacetal, or acetal.arrow_forwardClassify each alkyl halide as 1 °, 2 °, or 3 °.arrow_forward
- What carbonyl compound is needed to make each alcohol by a reduction reaction? What carbonyl compound is needed to make each alcohol by a reduction reaction?arrow_forward4. Complete the following reaction. reagent X H. HCN a) A в reagent Y b) OHarrow_forwardDraw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. c. CH3CH2Cl, AlCl3 l. product in (c), then KMnO4arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY