
Concept explainers
a.
Interpretation:
For the below compound, name has to be determined.
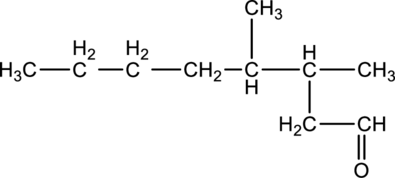
Concept Introduction:
Nomenclature of
Firstly, the
b.
Interpretation:
For the below compound, name has to be determined.
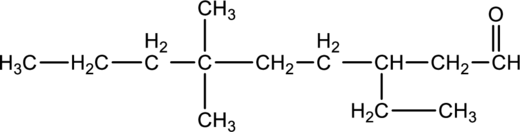
Concept Introduction:
Refer to part “a.”.
c.
Interpretation:
For the below compound, name has to be determined.
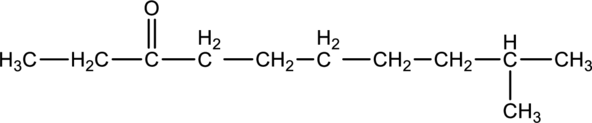
Concept introduction:
Naming of
Firstly, the functional group from the below structure will be identified as ketone and –one is added as the suffix. Subsequently find the longest carbon chain, which is bonded with the ketone group. In case of the cyclic compounds, numbering starts with ketone group attached carbon, which is considered a primary carbon. Prefix changes if any other substituents are present in the structure other than ketone group. In case of more than one substituents naming will be given in alphabetical order.
d.
Interpretation:
For the below compound, name has to be determined.
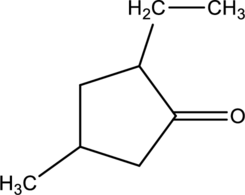
Concept Introduction:
Refer to part “c.”.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
- Interpreting NMR spectra is a skill that often requires some amount of practice, which, in turn, necessitates access to a collection of NMR spectra. Beyond Labz Organic Synthesis and Organic Qualitative Analysis have spectral libraries containing over 700 1H NMR spectra. In this assignment, you will take advantage of this by first predicting the NMR spectra for two closely related compounds and then checking your predictions by looking up the actual spectra in the spectra library. After completing this assignment, you may wish to select other compounds for additional practice. 1. Write the IUPAC names for the following two structures: Question 2 Question 3 2. Predict the NMR spectra for each of these two compounds by listing, in the NMR tables below, the chemical shift, the splitting, and the number of hydrogens associated with each predicted peak. Sort the peaks from largest chemical shift to lowest. **Not all slots must be filled**arrow_forward11:14 ... worksheets.beyondlabz.com 3. To check your predictions, click this link for Interpreting NMR Spectra 1. You will see a list of all the - compounds in the spectra library in alphabetical order by IUPAC name. Hovering over a name in the list will show the structure on the chalkboard. The four buttons on the top of the Spectra tab in the tray are used to select the different spectroscopic techniques for the selected compound. Make sure the NMR button has been selected. 4. Scroll through the list of names to find the names for the two compounds you have been given and click on the name to display the NMR spectrum for each. In the NMR tables below, list the chemical shift, the splitting, and the number of hydrogens associated with each peak for each compound. Compare your answers to your predictions. **Not all slots must be filled** Peak Chemical Shift (d) Multiplicity 1 2 3 4 5arrow_forwardО δα HO- H -Br δα HO-- + + -Br [B] 8+ HO- -Br δα नarrow_forward
- 1/2 - 51% + » GAY Organic Reactions Assignment /26 Write the type of reaction that is occurring on the line provided then complete the reaction. Only include the major products and any byproducts (e.g. H₂O) but no minor products. Please use either full structural diagrams or the combination method shown in the lesson. Skeletal/line diagrams will not be accepted. H3C 1. 2. CH3 A Acid OH Type of Reaction: NH Type of Reaction: + H₂O Catalyst + HBr 3. Type of Reaction: H3C 4. Type Reaction: 5. H3C CH2 + H2O OH + [0] CH3 Type of Reaction: 6. OH CH3 HO CH3 + Type of Reaction: 7. Type of Reaction: + [H]arrow_forwardhumbnai Concentration Terms[1].pdf ox + New Home Edit Sign in Comment Convert Page Fill & Sign Protect Tools Batch +WPS A Free Trial Share Inter Concreting Concentration forms. Hydrogen peroxide is a powerful oxidizing agent wed in concentrated solution in rocket fuels and in dilute solution as a hair bleach. An aqueous sulation of H2O2 is 30% by mass and has density of #liligime calculat the Ⓒmolality ⑥mole fraction of molarity. 20 9. B. A sample of Commercial Concentrated hydrochloric ETarrow_forwardIf a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forward
- Would the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward(a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forwardDraw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

