
Concept explainers
a.
Interpretation:
The product obtained when the below compound reacts with H2SO4 reagent has to be determined.
Concept introduction:
Dehydration of alcohol:
When alcohol reacts with
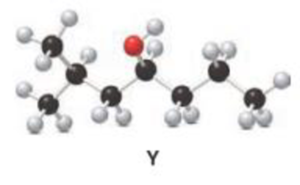
b.
Interpretation:
For a given Y is treated with
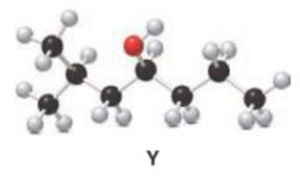
Concept introduction:
Oxidation of alcohol:
During oxidation of alcohol, the number of
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
- If a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardPlease help me answer the following questions using the data I included. 1&2arrow_forwardAssign all the Protons in HNMRarrow_forward
- PQ-10. What is the major product of this reaction? (A) (C) 930 Me HO O=S=O O-8-CF, C 어 Me H+ OH 270 O 0-5-0 O=S=O O-S-CF CF3 2arrow_forwardPredict the major organic product(s) of the following reactions. Include stereochemistry when necessary. Write NR if no reaction, try to explain.arrow_forwardQ2: Explain why epoxides that react in an SN1 manner will not show any stereochemical inversion in the product. Q3: Rationalize why Alcohol B will react under the indicated reaction conditions, but Alcohol A will not. A ☑ OH B OH PBr3 R-Brarrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


