
Answer the following questions about alcohol B.
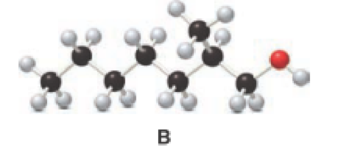
- a. Give the IUPAC name.
- b. Classify the alcohol as 1°, 2°, or 3°.
- c. Draw the products formed when B is dehydrated with H2SO4.
- d. What product is formed when B is oxidized with K2Cr2O7?
- e. Draw a constitutional isomer of B that contains an OH group.
- f. Draw a constitutional isomer of B that contains an ether.
a.
Interpretation:
IUPAC name for a given alcohol compound has to be determined.
Concept introduction:
Nomenclature of alcohol:
Firstly, find the longest carbon chain, which is bonded with the -OH group. The alcohol has to be identified and named as –ol, in the suffix. In case of cyclic compounds, numbering should be given from -OH group-attached carbon, which is considered as the lowest carbon. If any substituents other than -OH group are present, it is necessary to mention their name in prefix and if more than one substituents are present, then naming should be in alphabetical order. In the name of the parent carbon chain ‘e’ is replaced by –ol. If more than, one alcohol is present then it is named as di, tri, tetra, etc after the parent name but before the –ol.
Explanation of Solution
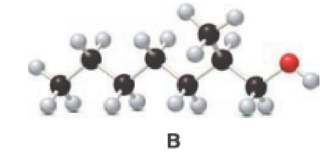
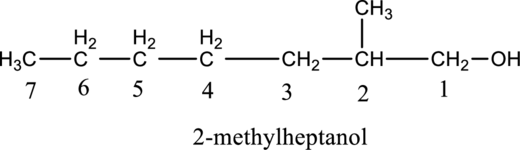
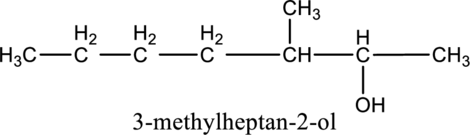
Given compound B is,
Step 1: Step 1: In a given compound the black, white and red color balls indicates carbon, hydrogen and oxygen atoms. In the given compound the longest carbon chain, which is attached to the OH group is found and numbering is given from OH group-attached carbon. The OH group-attached carbon is considered as a lowest numbering.
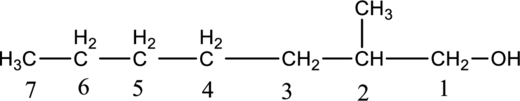
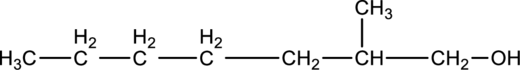
Step 2: For naming the compound, firstly, the parent carbon chain should be checked, and as it possess seven carbon atoms, it is named as heptane. Due to -OH group present in the compound the last letter from the parent carbon naming ‘e’ is replaced with -ol. As one methyl group is present in the second position of parent chain, it is named as 2-methyl. Finally, the compound name is 2-methylheptanol.
b.
Interpretation:
From the compound B, the type of alcohol has to be determined.
Concept introduction:
Classification of alcohol:
Generally, the alcohol group is bonded with minimum one alkyl group. The alcohol group is classified into three types, such as primary (1∘), secondary (2∘) and tertiary (3∘). It depends on number of carbons attached to the single carbon atom bonded with the alcohol group. Primary (1∘) alcohol contains one carbon bonded with alcohol attached carbon atom. Secondary (2∘) alcohol contains two carbon bonded with alcohol attached carbon atom. Tertiary (3∘) alcohol contains three carbon bonded with alcohol attached carbon atom.
Explanation of Solution
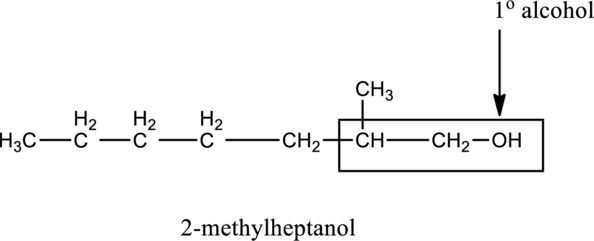
In the below compound A, OH group attached carbon atom is bonded with one carbon atom, therefore it is primary (1∘) alcohol.
c.
Interpretation:
The product obtained when the compound B reacts with H2SO4 reagent has to be determined.
Concept introduction:
Dehydration of alcohol:
When alcohol reacts with H2SO4, it gives alkene as a product. This reaction involves elimination of water molecule by breaking of bonds between two adjacent atoms (C-OH and C-H), which is called as a dehydration reaction.
Explanation of Solution
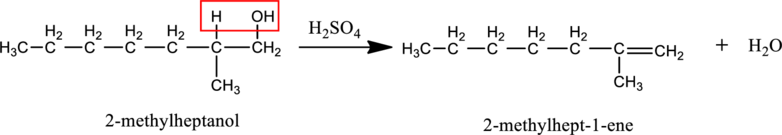
Given stating material B is 2-methylheptanol (1o alcohol),
When 2-methylheptanol reacts with H2SO4, elimination of water molecule from reactant and double bond formation on product side takes place resulting into 2-methylhept-1-ene as product as shown below.
d.
Interpretation:
The product obtained when the compound B reacts with K2Cr2O7 reagent has to be determined.
Concept introduction:
Oxidation of alcohol:
During oxidation of alcohol, the number of C-O bonds gets increased on product side and C-H bond gets decreased on reactant side. In the oxidation reaction, reaction takes place between carbon attached to hydrogen atom and carbon attached to OH group and finally C-O bond is formed leading to H2 removal from the reactant. Oxidation of an alcohol gives different products depending upon the type of alcohols and reagents take part in a reaction. Oxidation of alcohols give carbonyl group in the product side. In a primary alcohol, among the two C-H bonds present, the first C-H bond gets oxidized into aldehyde group and then further it gets oxidized into carboxylic acid group. In case of the secondary alcohol, only the one C-H bond present is oxidized into ketones. For tertiary alcohol, there are no hydrogen atoms present and therefore, tertiary alcohols are not been able to be oxidized.
Explanation of Solution
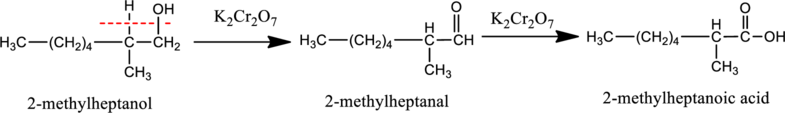
Given stating material A is 2-methylheptanol (1o alcohol),
When 2-methylheptanol, the primary alcohol containing two hydrogen atom on the carbon attached with OH group reacts with K2Cr2O7.. Therefore, it is first oxidized to give 2-methylheptanal and then the 2-methylheptanal is further oxidized into 2-methylheptanoic acid as shown below.
e.
Interpretation:
The constitutional isomer of compound B that contain OH group has to be determined.
Concept introduction:
Constitutional isomers:
The compounds, that contain same molecular formula but different with respect to molecular orientation. In other words compounds having similar molecular formula and different structure are called as Constitutional isomers.
Explanation of Solution
In the given compound B molecular formula is C8H18O, it contains one OH group. This compound has eighteen constitutional isomer as given below.
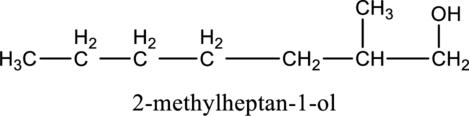
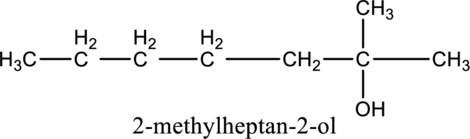
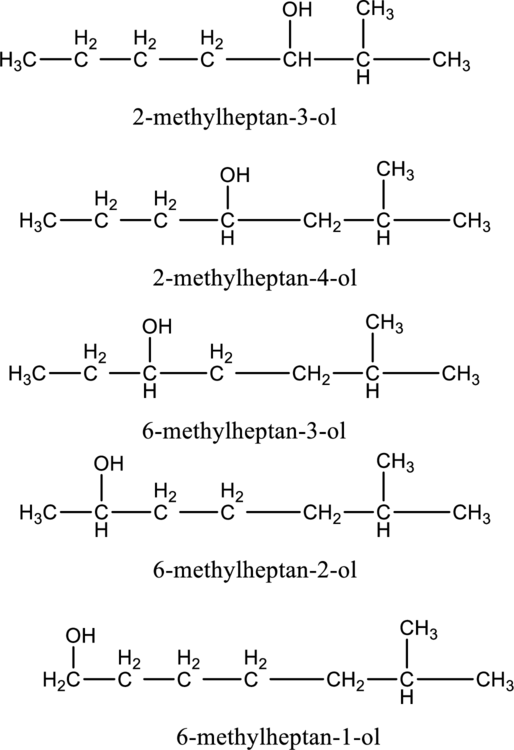
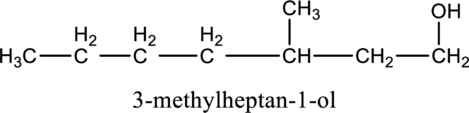
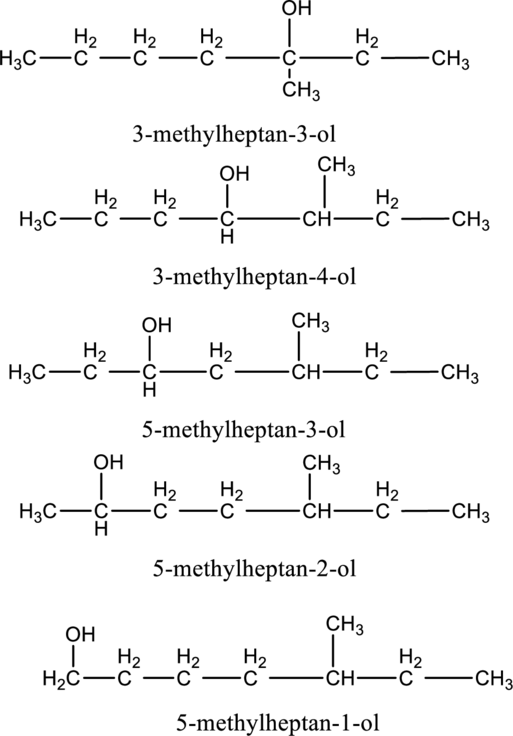
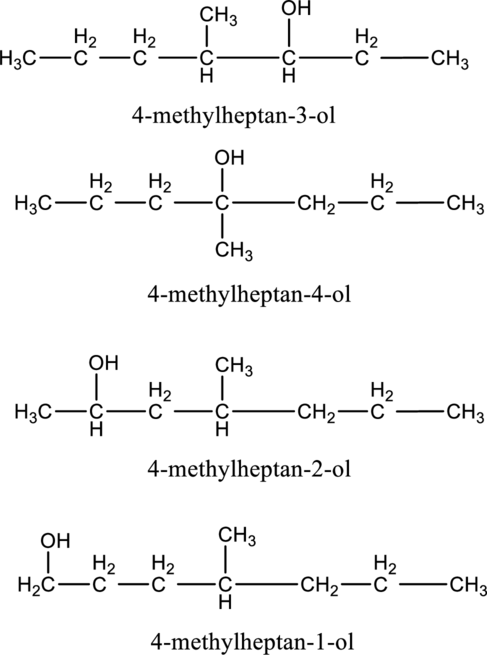
f.
Interpretation:
The constitutional isomer of compound B that contain an ether group has to be determined.
Concept introduction:
Refer to part ‘e.’.
Explanation of Solution
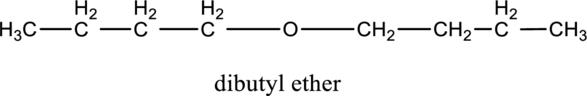
In the given compound A molecular formula is C8H18O, it contains one O atom. Therefore, four constitutional isomer of ether containing compound is possible and it as given below.
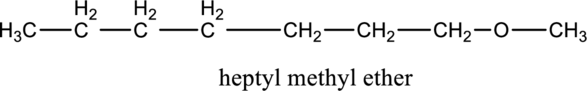
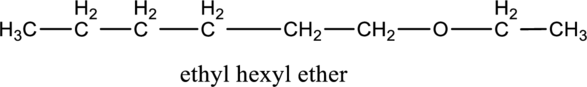
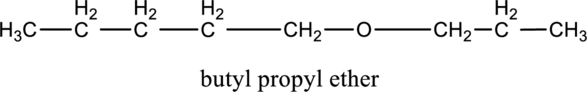
Want to see more full solutions like this?
Chapter 12 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
- Can you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forward
- consider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
- What is the organic molecule X of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat are is the organic molecule X and product Y of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Without using graphs, calculate the order of the reaction. t/s [R]/(mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forward
- Predict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat are the products of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





