
Concept explainers
a.
Interpretation:
For the below compound, name has to be determined.
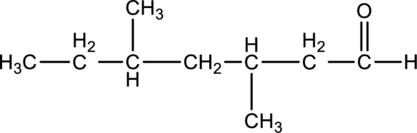
Concept Introduction:
Nomenclature of
Firstly, the
b.
Interpretation:
For the below compound, name has to be determined.
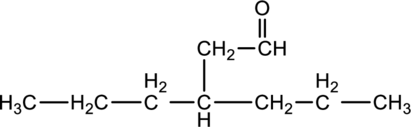
Concept Introduction:
Refer to part “a.”.
c.
Interpretation:
For the below compound, name has to be determined.
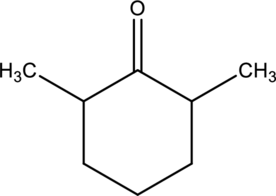
Concept introduction:
Naming of
Firstly, the functional group from the below structure will be identified as ketone and –one is added as the suffix. Subsequently find the longest carbon chain, which is bonded with the ketone group. In case of the cyclic compounds, numbering starts with ketone group attached carbon, which is considered a primary carbon. Prefix changes if any other substituents are present in the structure other than ketone group. In case of more than one substituents naming will be given in alphabetical order.
d.
Interpretation:
For the below compound, name has to be determined.
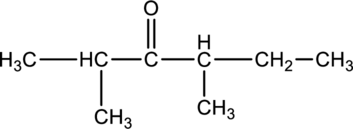
Concept Introduction:
Refer to part “c.”.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
- A student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forwardCalculate the density of 21.12 g of an object that displaces 0.0250 L of water.arrow_forward
- Draw the expected reactant R28. Cu(II) CO₂Mearrow_forwardPpplllleeeaaasssseeee helllppp wiithhh thisss Organic chemistryyyyyy I talked like this because AI is very annoyingarrow_forwardName the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward
- 1b. Br LOHarrow_forwardI would like my graphs checked please. Do they look right? Do I have iodine and persulfate on the right axis ?arrow_forwardReaction Fill-ins Part 2! Predict the product(s) OR starting material of the following reactions. Remember, Hydride shifts are possible if/when a more stable carbocation can exist (depending on reaction mechanism)! Put your answers in the indicated boxes d. d. ง HCIarrow_forward
- A cylinder contains 12 L of water vapour at 150˚C and 5 atm. The temperature of the water vapour is raised to 175˚C, and the volume of the cylinder is reduced to 8.5 L. What is the final pressure of the gas in atmospheres? assume that the gas is idealarrow_forwardOn the next page is an LC separation of the parabens found in baby wash. Parabens are suspected in a link to breast cancer therefore an accurate way to quantitate them is desired. a. In the chromatogram, estimate k' for ethyl paraben. Clearly indicate what values you used for all the terms in your calculation. b. Is this a "good" value for a capacity factor? Explain. c. What is the resolution between n-Propyl paraben and n-Butyl paraben? Again, indicate clearly what values you used in your calculation. MAU | Methyl paraben 40 20 0 -2 Ethyl paraben n-Propyl paraben n-Butyl paraben App ID 22925 6 8 minarrow_forwardd. In Figure 4, each stationary phase shows some negative correlation between plate count and retention factor. In other words, as k' increases, N decreases. Explain this relationship between k' and N. Plate Count (N) 4000 3500 2500 2000 1500 1000 Figure 4. Column efficiency (N) vs retention factor (k') for 22 nonionizable solutes on FMS (red), PGC (black), and COZ (green). 3000 Eluent compositions (acetonitrile/water, A/W) were adjusted to obtain k' less than 15, which was achieved for most solutes as follows: FMS (30/70 A/W), PGC (60/40), COZ (80/20). Slightly different compositions were used for the most highly retained solutes. All columns were 50 mm × 4.6 mm id and packed with 5 um particles, except for COZ, which was packed with 3 um particles. All other chromatographic conditions were constant: column length 5 cm, column j.§. 4.6 mm, flow rate 2 mL/min, column temperature 40 °C, and injection volume 0.5 μL Log(k'x/K'ethylbenzene) FMS 1.5 1.0 0.5 0.0 ཐྭ ཋ ཤྩ བྷྲ ; 500 0 5 10…arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning



