
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.36UKC
Consider the following ball-and-stick model.
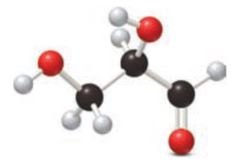
- a. Locate the chirality center.
- b. Is the carbonyl group part of an
aldehyde or aketone ? - c. Classify each hydroxyl group as 1°, 2°, or 3°.
- d. What product is formed when this compound is treated with Tollens reagent?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Answer the following questions about the carbohydrate erythrulose, an ingredient in sunless tanning agents. erythrulose a. Does erythrulose contain an aldehyde or ketone? b. Classify each hydroxyl group as 1 °, 2 °, or 3 °. c. What product is formed when erythrulose is treated with Tollens reagent? d. What product is formed when erythrulose is treated with K 2Cr 2O 7?
Reactions of carbonyl compounds with hydride ion donors.
a. Draw reaction of an aldehyde with sodium borohydride forms a primary alcohol.
b. Draw reaction of a ketone with sodium borohydride forms a secondary alcohol.
Which is propyl propanoate?
A. CH₂CH₂CH₂OOCCH₂CH;
B. CH₂CH₂CH₂COOCH₂CH₂
C. CH₂CH₂CH₂COCH₂CH₂
D. CHỊCH,CH,OCH,CHỊCH,
A
B
C
D
Chapter 12 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 12.1 - a. Label the hydroxyl groups, thiols, halogens,...Ch. 12.1 - Draw out each compound to clearly show what groups...Ch. 12.2 - Classify each alcohol as 1, 2, or 3.Ch. 12.2 - Classify each hydroxyl group in sorbitol as 1, 2,...Ch. 12.2 - Which compound in each pair has the higher boiling...Ch. 12.2 - Label each compound as water soluble or water...Ch. 12.2 - Give the IUPAC name for each compound.Ch. 12.2 - Give the structure corresponding to each name. a....Ch. 12.3 - Name each ether. a. CH3OCH2CH2CH2CH3 b....Ch. 12.3 - Prob. 12.10P
Ch. 12.3 - Which compound in each pair has the higher boiling...Ch. 12.5 - Prob. 12.12PCh. 12.5 - Prob. 12.13PCh. 12.6 - Prob. 12.14PCh. 12.6 - Prob. 12.15PCh. 12.6 - Give the structure corresponding to each name. a....Ch. 12.7 - Prob. 12.17PCh. 12.8 - Give the IUPAC name for each aldehyde. a....Ch. 12.8 - Prob. 12.19PCh. 12.8 - Give the IUPAC name for each aldehyde depicted in...Ch. 12.8 - Prob. 12.21PCh. 12.8 - Prob. 12.22PCh. 12.8 - Acetone and progesterone are two ketones that...Ch. 12.9 - Prob. 12.24PCh. 12.10 - Prob. 12.25PCh. 12.11 - Prob. 12.26PCh. 12.11 - Prob. 12.27PCh. 12.11 - Prob. 12.28PCh. 12.11 - Prob. 12.29PCh. 12.11 - Prob. 12.30PCh. 12.11 - Prob. 12.31PCh. 12.11 - Prob. 12.32PCh. 12 - Prob. 12.33UKCCh. 12 - Prob. 12.34UKCCh. 12 - Consider the following ball-and-stick model of an...Ch. 12 - Consider the following ball-and-stick model. a....Ch. 12 - Name each compound. a. CH3CH2OCH2CH2CH2CH3Ch. 12 - Name each compound. a. CH3OCH2CH2CH3 b....Ch. 12 - Answer the following questions about alcohol A. a....Ch. 12 - Answer the following questions about alcohol B. a....Ch. 12 - Prob. 12.41UKCCh. 12 - Prob. 12.42UKCCh. 12 - Prob. 12.43UKCCh. 12 - Prob. 12.44UKCCh. 12 - Prob. 12.45APCh. 12 - Prob. 12.46APCh. 12 - Prob. 12.47APCh. 12 - Prob. 12.48APCh. 12 - Prob. 12.49APCh. 12 - Prob. 12.50APCh. 12 - Prob. 12.51APCh. 12 - Prob. 12.52APCh. 12 - Prob. 12.53APCh. 12 - Give the structure corresponding to each name. a....Ch. 12 - Prob. 12.55APCh. 12 - Draw structures for the four constitutional...Ch. 12 - Prob. 12.57APCh. 12 - Rank the following compounds in order of...Ch. 12 - Explain why two four-carbon organic molecules have...Ch. 12 - Explain why the boiling point of CH3CH2CH2CH2OH...Ch. 12 - Which compound in each pair has the higher boiling...Ch. 12 - Which compound in each pair is more water soluble?...Ch. 12 - Prob. 12.63APCh. 12 - Prob. 12.64APCh. 12 - Prob. 12.65APCh. 12 - Prob. 12.66APCh. 12 - Prob. 12.67APCh. 12 - Xylitol is a nontoxic compound as sweet as table...Ch. 12 - Prob. 12.69APCh. 12 - Prob. 12.70APCh. 12 - Prob. 12.71APCh. 12 - Prob. 12.72APCh. 12 - Prob. 12.73APCh. 12 - Prob. 12.74APCh. 12 - Prob. 12.75APCh. 12 - Prob. 12.76APCh. 12 - Prob. 12.77APCh. 12 - Draw the structure corresponding to each name. a....Ch. 12 - Prob. 12.79APCh. 12 - Prob. 12.80APCh. 12 - What product is formed when each compound is...Ch. 12 - Prob. 12.82APCh. 12 - Prob. 12.83APCh. 12 - Prob. 12.84APCh. 12 - Prob. 12.85APCh. 12 - Prob. 12.86APCh. 12 - Prob. 12.87APCh. 12 - Label each of the following objects as chiral or...Ch. 12 - Prob. 12.89APCh. 12 - Prob. 12.90APCh. 12 - Prob. 12.91APCh. 12 - Prob. 12.92APCh. 12 - Prob. 12.93APCh. 12 - Prob. 12.94APCh. 12 - Prob. 12.95APCh. 12 - Prob. 12.96APCh. 12 - Prob. 12.97APCh. 12 - How are the compounds in each pair related? Are...Ch. 12 - Prob. 12.99APCh. 12 - Prob. 12.100APCh. 12 - Prob. 12.101APCh. 12 - Prob. 12.102APCh. 12 - Prob. 12.103APCh. 12 - Lactic acid [CH3CH(OH)CO2H] gives sour milk its...Ch. 12 - Prob. 12.105APCh. 12 - Prob. 12.106APCh. 12 - Prob. 12.107CPCh. 12 - Prob. 12.108CPCh. 12 - Prob. 12.109BTCCh. 12 - Prob. 12.110BTC
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. d. (CH3CH2)2CHCOCl, AlCl3 j. product in (d), then NH2NH2, – OHarrow_forwardDraw the product formed when pentanal (CH3 CH₂ CH₂ CH₂ CHO) is treated with each reagent. With some reagents, no reaction occurs. a. NaBH4, CH3OH b. [1] LiAiH4: [2] H₂O c. H₂, Pd-C d. PCC e. Na₂Cr₂O7, H₂SO4, H₂O f. Ag₂O, NH4OH g. [1] CH3 MgBr; [2] H₂O h. [1] C6H5 Li: [2] H₂O i. [1] (CH3)2 CuLi; [2] H₂O J. [1] HC=CNa; [2] H₂O k. [1] CH 3 C=CLI; [2] H₂O 1. The product in (a), then TBDMS-CI, imidazolearrow_forwardDraw the products formed when each alcohol undergoes dehydration with TsOH, and label the major product when a mixture results.arrow_forward
- Amino acids such as glycine are the building blocks of large molecules called proteins that give structure to muscle, tendon, hair, and nails. a. Explain why glycine does not actually exist in the form with all atoms uncharged, but actually exists as a salt called a zwitterion. b. What product is formed when glycine is treated with concentrated HCl? c. What product is formed when glycine is treated with NaOH?arrow_forwardGive the IUPAC name (including any E,Z designation) for each unsaturated aldehyde.arrow_forwardCompare aldehydes and ketones as to (Use acetaldehyde and acetone as examples). 1. Reaction with Tollen’s reagent 2. Reaction with Fehling’s reagent 3. Reaction with dilute NaOHarrow_forward
- 1. What are the characteristics of a positive tollens test for adehydes? What is the oxidizing agent in tollens solutions? 2. What is the characteristics of a positive Benedict's test for aldehydes? What is the oxidizing agent in Benedict's solution?arrow_forwardDraw the missing starting material. Reagent 1 is benzene and AlCl3. Reagent B is Zn(Hg) and HCl. Th structure is not isobutyraldehydearrow_forwardInstructions: Draw out each compound to clearly show what groups are bonded to the carbonyl carbon. Label each compound as a carboxylic acid, ester, or amide. a. CH3CH2CO2CH2CH3 b. CH3CONHCH3 c. (CH3)3CCO2H d. (CH3)2CHCON(CH3)2 Instructions: Give the IUPAC name for each compound. A. CH₂ CH₂CH₂CH₂CCH₂COOH CH3 B. CH₂CHCH₂CH₂COOH CH₂COOH CH₂CH3 C. (CH,CH,),CHCH,CHCOOH Instructions: Give the structure corresponding to each IUPAC name. a. 2-bromobutanoic acid b. 2,3-dimethylpentanoic acid c. 2-ethyl-5,5-dimethyloctanoic acid d. 3,4,5,6-tetraethyldecanoic acidarrow_forward
- Draw the eight constitutional isomers having the molecular formula C5H11Cl.a. Give the IUPAC name for each compound (ignoring R and S designations).b. Classify each alkyl halide as 1°, 2°, or 3°.c. Label any stereogenic centers.d. For each constitutional isomer that contains a stereogenic center, draw all possible stereoisomers, and label each stereogenic center as R or S.arrow_forwardDraw the eight constitutional isomers having the molecular formula C5H11Cl. a.Give the IUPAC name for each compound (ignoring R and S designations). b.Classify each alkyl halide as 1°, 2°, or 3°. c.Label any stereogenic centers. d.For each constitutional isomer that contains a stereogenic center, draw all possible stereoisomers, and label each stereogenic center as R or S.arrow_forwardWhich of the following are a. hemiacetals? b. acetals? c. hydrates?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY