
a.
Interpretation:
For a below ball and stick model, nomenclature has to be determined.
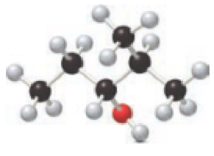
Concept introduction:
Nomenclature of organic compounds:
Firstly, the
For halide compounds, longest carbon chain attached with the halogen atom has to be identified and the compound will be named as–‘ine’ to –‘o’. Ex: Chlorine to chloro. If any other substituents are present, then it is necessary to mention of the substituent number. Finally, names of all the substituents will be arranged in alphabetical order including halogen atoms, their position and followed by parent alkane chain name at the end.
Nomenclature of halide:
b.
Interpretation:
For a given ball and stick model, name has to be determined and the respective structure is given below.
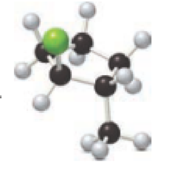
Concept introduction:
Refer to part “a.”.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
- Benzoic acid is used to determine the heat capacity of bomb calorimeters because it can be obtained in pure form and its energy of combustion is known very accurately (−26.43 kJ/g). Determine the heat capacity of a calorimeter that had a temperature increase of 9.199°C when 3.500 g of benzoic acid was used.arrow_forwardGiven the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. 2N2H4(g) + 2NO2(g) → 3N2(g) + 4H2O(g) AHrxn ? kJ Substance AH in kJ/mol N2H4(g) +95.4 NO2(g) +33.1 H2O(g) -241.8arrow_forwardIf 7.3 kJ of energy are required to change the temperature of water from 5.0 to 70.0, what was the volume of water? (cs = 4.184 J/(g ⋅ ), d = 1.00 g/mL)arrow_forward
- BALANCE CHEMICAL REACTIONarrow_forwardPredict the product(s) of the following reactions. If no reaction, write "NR". a) Cl₂ FeCl3 e) HNO3 H2SO4 b) NO2 CI. HNO3 f) Br Br2 OH H2SO4 HO3S. FeBr3 c) Cl2 g) FeCl3 F d) O₂N Br2 FeBr3 O₂N OH HNO3 CH3 H2SO4arrow_forwardulating the pH salt solution Calculate the pH at 25 °C of a 0.75M solution of anilinium chloride (C6H5NH3C1). Note that aniline (C6H5NH2) is a weak base with a pK of 4.87. Round your answer to 1 decimal place. pH = ☐ ☑ ⑤ ? olo 18 Ararrow_forward
- I apologize, but the app is not allowing me to post the other 4 pictures of the thermodynamics chart. But I believe the values are universal. Please help!arrow_forwardCalculating the pH of a salt solution Calculate the pH at 25 °C of a 0.29M solution of potassium butanoate (KC3H,CO2). Note that butanoic acid (HC3H,CO2) is a weak acid with a pKa of 4.82. Round your answer to 1 decimal place. pH = -0 Х olo 18 Ararrow_forward: At a certain temperature, the equilibrium constant K for the following reaction is 1.58 × 10-12 N2(g) + O2(g) = 2 NO(g) Use this information to complete the following table. Suppose a 38. L reaction vessel is filled with 0.93 mol of N2 and 0.93 mol of O2. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little N2 and O2. There will be very little NO. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 NO(g) N2(9)+02(9) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 3 N2(9)+302(g) 6 NO(g) Neither of the above is true. K = ☐ K = ☐ ☐ X10 Х D ? 000 18 Ar Barrow_forward
- when performing the reaction that involves 2 equivalents of 3-(diethylamino)-phenol and Phthalic anhydride with sulfuric acid and water react to form rhodamine b where the Phthalic anhydride cleaves in acid and how does Excessive Washing (w/ Base) & Subsequent Resonance Structure get affectedarrow_forward3. The strongest acid of the following compounds is ___.A. p-nitrophenol; B. m-nitrophenol; C. o-chlorophenol;D. p-methoxyphenol; E. o-methylphenol Please explain your steps and thought process. Thank you!arrow_forwardUsing the general properties of equilibrium constants At a certain temperature, the equilibrium constant K for the following reaction is 1.3 × 10 4: Cl2(g) + CHCl3(g) HCl(g) + CC₁(g) Use this information to complete the following table. Suppose a 16. L reaction vessel is filled with 1.6 mol of HCI and 1.6 mol of CCl4. What can you say about the composition of the mixture in the vessel at equilibrium? There will be very little Cl2 and CHCl3. ☐ x10 There will be very little HCI and CCl4. Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. HCl(g)+CC14(g) 12 Cl2(9)+CHCl3(9) K = 0 ☐ What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. 2 Cl₂(9)+2CHCl3(9) 2 HCl(9)+2CC₁₁(9) K = ✓ 00. 18 Ararrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





