
Concept explainers
Interpretation: Whether the volume of a gas can be decreased to zero or not is to be explained.
Concept introduction: Matter is said to be anything that has the ability to occupy volume and has mass also.
Answer to Problem 5E
The volume of the gas cannot be zero. Gas is a matter and thus possesses the property of mass and occupies space.
Explanation of Solution
A decrease in temperature of the gas leads to a point where it converts to liquid state. The volume change becomes negligible on conversion of gas to liquid state. On further decrease in temperature, the gas further converts to solid state. Thus, the gas can never attain volume of zero. The gas is converted into the liquid state at a volume having value greater than zero. On extrapolating the graph in the opposite direction, it intersects the x-axis at a temperature of
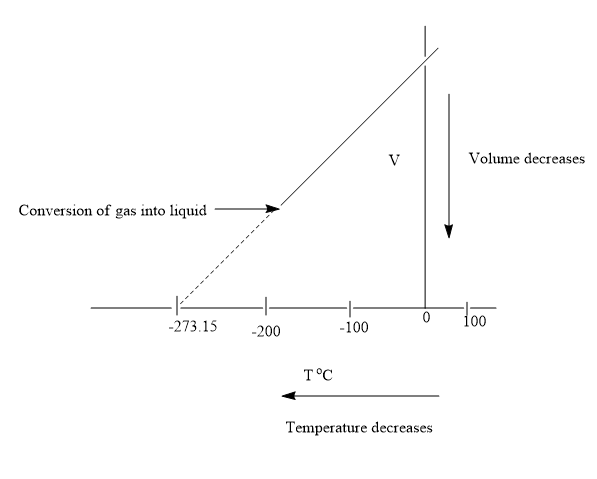
In case the gas has not converted to liquid state then at this temperature the volume of the gas would have become zero. But in actual life, the gas is considered to be matter and matter has a property of occupying space.
The volume of the gas cannot be zero. Gas is a matter and thus possesses the property of mass and occupies space.
Chapter U3 Solutions
Living by Chemistry
Additional Science Textbook Solutions
Anatomy & Physiology (6th Edition)
Campbell Essential Biology (7th Edition)
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Applications and Investigations in Earth Science (9th Edition)
Organic Chemistry (8th Edition)
Cosmic Perspective Fundamentals
- Draw the mechanism (including all curved arrows for electron movement) showing how the maleicanhydride is attacked by the anthracene and formation of the final Diels Alder product.arrow_forwardProvide the missing information. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward
- Provide the missing information. *see imagearrow_forwardI have a bottle of butanal that has been improperly used by lab workers. They allowed a traceamount NaOH (aq) to contaminate the bottle. What is now in my bottle of “butanal? What is the molecular name and functional group name? Draw the structure.arrow_forwardProvide the missing information. *see imagearrow_forward
- First image: Why can't the molecule C be formed in those conditions Second image: Synthesis for lactone C its not an examarrow_forwardFirst image: I have to show the mecanism for the reaction on the left, where the alcohol A is added fast in one portion Second image: I have to show the mecanism of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primaryarrow_forwardFirst image: I have to explain why the molecule C is never formed in those conditions. Second image: I have to propose a synthesis for the lactone Aarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





