
(a)
Interpretation: The incorrect bonds in the given electron dot structure of
Concept Introduction: An electron dot structure represents the arrangement of total valence electrons in a molecule. Here, electrons are represented as dots in pairs around the symbol of atoms of the molecule.
(a)
Explanation of Solution
The given molecule is
. The structure given in the question is as follows:
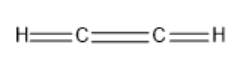
Here, C can maximum form 4 bonds and H can maximum of 1 bond. Hydrogen cannot form a double bond with any atom of the element. Thus, it will be C-H single bonds. Now, each carbon must form 4 bonds thus, it will C-C triple bond instead of a double bond. Also, in an electron dot structure, a binding pair of electrons and lone pair of electrons are shown around the atoms. The correct electron dot structure can be represented as follows:
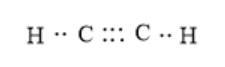
(b)
Interpretation: The incorrect bonds in the given electron dot structure of
Concept Introduction: An electron dot structure represents the arrangement of total valence electrons in a molecule. Here, electrons are represented as dots in pairs around the symbol of atoms of the molecule.
(b)
Explanation of Solution
The given molecule is FOH, the given electron dot structure is as follows:
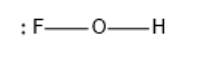
In the above structure, F is singly bonded to an oxygen atom which is then bonded to a hydrogen atom. Here, the octet for F, O, and H is complete but all the lone pairs of electrons are not represented.
Here, F has 7 valence electrons thus, there should be 3 lone pairs of electrons on the F atom. Similarly, the valence electrons of the oxygen atom are 6 and it should have 2 lone pairs of electrons. The correct electron dot structure is as follows:
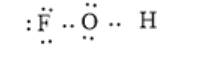
(c)
Interpretation: The incorrect bonds in the given electron dot structure of
Concept Introduction: An electron dot structure represents the arrangement of total valence electrons in a molecule. Here, electrons are represented as dots in pairs around the symbol of atoms of the molecule.
(c)
Explanation of Solution
The given molecule is ICl, the given electron dot structure is as follows:
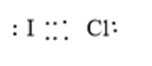
Here, I and Cl both have 7 valence electrons thus, each needs one electron to complete the octet. Iodine and Chlorine can share one electron each to form a covalent bond. Since one bond pair is shared thus, a single bond is formed between I and Cl. Moreover, there will be 3 lone pairs of electron/s each on I and Cl atoms. The correct electron dot structure can be represented as follows:
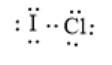
(d)
Interpretation: The incorrect bonds in the given electron dot structure of
Concept Introduction: An electron dot structure represents the arrangement of total valence electrons in a molecule. Here, electrons are represented as dots in pairs around the symbol of atoms of the molecule.
(d)
Explanation of Solution
The given molecule is
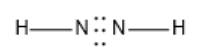
Here, the number of valence electrons in the N and H atoms is 5 and 1 respectively. Here, the octet of N is not completed. H atom can maximum share one electron thus, only a single bond is possible. After the formation of single bonds with N, there will be 4 electrons in each N atom. Thus, it will have an N-N double bond. Also, there will be 1 lone pair of electrons on each N atom.
The correct electron dot structure is represented as follows:
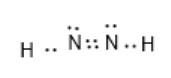
Chapter 8 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
- can someone give a description of this NMR including whether its a triplt singlet doublet where the peak is around at ppm and what functional group it representsarrow_forward1. Determine the relationship between the following molecules as identical, diastereomers, or enantiomers (6 points, 2 points each). OH OH OH A-A OH HOT HO- ACHN and HO- ACHN OH HO HO ° OH and OH OH SH and ...SHarrow_forward20,0 Complete the electron pushing mechanism to y drawing the necomery unicaciones and carved on for Step 1: Add curved arms for the tint step, traiment with NalilĻ. The Nation 458 Step 2: Added for the second step, inalment with), how the "counterion bar Step 3: Daw the products of the last simplom organic and one incoganic spacient, including all nonbondingarrow_forward
- please provide the structure for this problem, thank you!arrow_forwardDraw the Fischer projection from the skeletal structure shown below. HO OH OH OH OH H Q Drawing Atoms, Bonds and Rings Charges I ☐ T HO H H OH HO I CH2OH H OH Drag H OH -CH2OH CHO -COOH Undo Reset Remove Donearrow_forwardplease provide the structure for this problem, thank youarrow_forward
- presented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forwardReaction A 0,0arrow_forwardpresented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward
- 6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forwardpresented by Mr L How the coprion. (Il Done in no wraction, dew the starting redential) доarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





