
Interpretation: The electron dot structure for given molecules needs to be drawn.
Concept Introduction: An electron dot structure represents the arrangement of total valence electrons in a molecule. Here, electrons are represented as dots in pairs to show the lone pairs as well as the bond pairs.
Explanation of Solution
The molecules in the given example are as follows:
In
The valence electrons of C atom is 4 and that of H atom is 1. Thus, the total number of valence electrons will be:
The arrangement of electrons can be represented as follows:
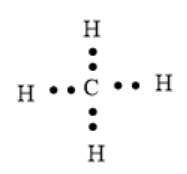
In
The number of valence electrons of N and Cl atom is 5 and 7 respectively. Thus, the total number of valence electrons will be:
The arrangement of electrons can be represented as follows:
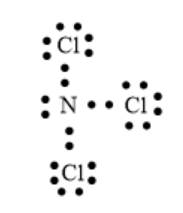
In
Here, S has 2 lone pairs of electrons.
The arrangement of electrons can be represented as follows:
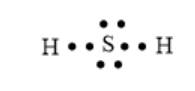
In the HF molecule, 1 H and 1 F atoms are bonded together via a single bond. The valence electrons of the H and F atom is 1 and 7 respectively. Thus, the total number of valence electrons will be 8. Here, F has 3 lone pairs of electrons. The arrangement of electrons can be represented as follows:
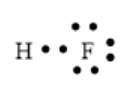
Chapter 8 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
- option choice: Isoleucine Histidine Threonine Alanine Lysine Aspartate Tryptophan Tyrosine Leucine Arginine Cysteine Asparagine Valine Glutamine Glycine Methionine Serine Proline Phenylalanine Glutamatearrow_forwardsketch the nature of the metal-alkylidene bonding interactions.arrow_forwardPart C The perspective formula of isoleucine, an amino acid, is provided below. HOOC H₂NIC H 川 CH3 CH,CH3 Draw the Newman projection in staggered conformation for isoleucine by viewing the molecule along the C-2-C-3 bond. 1. Edit the Newman projection on the canvas. 2. Replace the appropriate hydrogens with the appropriate -CH3 or other groups. 3. If you need to start over, Undo or choose a Newman projection from the Templates toolbar (bottom). Important: Never delete the hydrogen atoms or bonds directly attached to the template, and do not move them by dragging or dropping them. That will break the projections structures. Only replace them! ▸ View Available Hint(s) 0 2 H± 3D EXP. L ד י CONT. 2 H 0 N оarrow_forward
- Use the literature Ka value of the acetic acid, and the data below to answer these questions. Note: You will not use the experimental titration graphs to answer the questions that follow. Group #1: Buffer pH = 4.35 Group #2: Buffer pH = 4.70 Group #3: Buffer pH = 5.00 Group #4: Buffer pH = 5.30 Use the Henderson-Hasselbalch equation, the buffer pH provided and the literature pKa value of acetic acid to perform the following: a) calculate the ratios of [acetate]/[acetic acid] for each of the 4 groups buffer solutions above. b) using the calculated ratios, which group solution will provide the best optimal buffer (Hint: what [acetate]/[acetic acid] ratio value is expected for an optimal buffer?) c) explain your choicearrow_forwardHow would you prepare 1 liter of a 50 mM Phosphate buffer at pH 7.5 beginning with K3PO4 and 1 M HCl or 1 M NaOH? Please help and show calculations. Thank youarrow_forwardDraw the four most importantcontributing structures of the cation intermediate thatforms in the electrophilic chlorination of phenol,(C6H5OH) to form p-chlorophenol. Put a circle aroundthe best one. Can you please each step and also how you would approach a similar problem. Thank you!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





