
Concept explainers
(a)
Interpretation:
The following intermediate compound generated in the first or second cycle of the lipogenesis pathway is produced by (1) a dehydration reaction, (2) a hydrogenation reaction, or (3) a condensation reaction has to be identified.
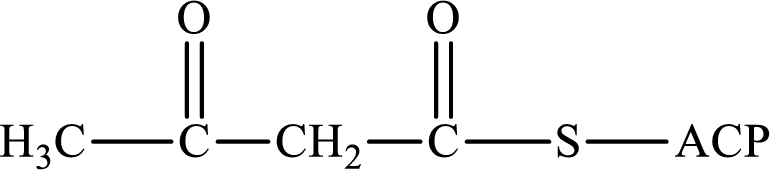
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part there is a cyclic process to synthesize saturated fatty acid.
The different reactions that are involved in the cyclic process are:

In hydrogenation reaction, a hydrogen molecule (H2) is added to an organic substance; in hydration reaction, a water molecule (H2O) is added to an unsaturated substrate; in the condensation reaction, two molecules combine to form a single product.
The first turn of the cyclic process produces four-carbon acyl group and the further turns add two carbon unit to the four-carbon acyl group. Therefore, the first turn has four carbon units and the second turn has six carbon unit in their intermediate compound.
(b)
Interpretation:
The following intermediate compound generated in the first or second cycle of the lipogenesis pathway is produced by (1) a dehydration reaction, (2) a hydrogenation reaction, or (3) a condensation reaction has to be identified.
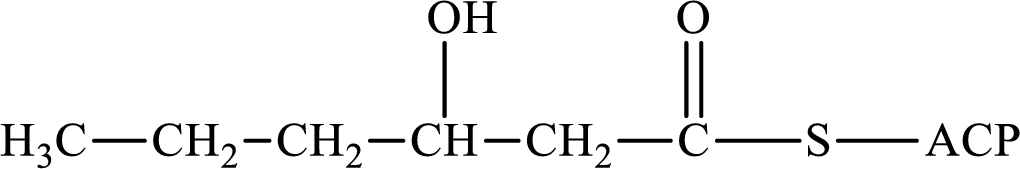
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part there is a cyclic process to synthesize saturated fatty acid.
The different reactions that are involved in the cyclic process are:

In hydrogenation reaction, a hydrogen molecule (H2) is added to an organic substance; in hydration reaction, a water molecule (H2O) is added to an unsaturated substrate; in the condensation reaction, two molecules combine to form a single product.
The first turn of the cyclic process produces four-carbon acyl group and the further turns add two carbon unit to the four-carbon acyl group. Therefore, the first turn has four carbon units and the second turn has six carbon unit in their intermediate compound.
(c)
Interpretation:
The following intermediate compound generated in the first or second cycle of the lipogenesis pathway is produced by (1) a dehydration reaction, (2) a hydrogenation reaction, or (3) a condensation reaction has to be identified.
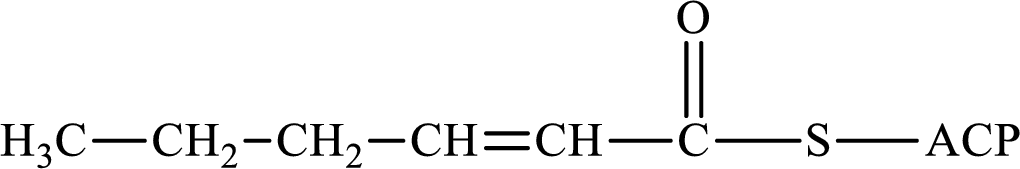
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part there is a cyclic process to synthesize saturated fatty acid.
The different reactions that are involved in the cyclic process are:

In hydrogenation reaction, a hydrogen molecule (H2) is added to an organic substance; in hydration reaction, a water molecule (H2O) is added to an unsaturated substrate; in the condensation reaction, two molecules combine to form a single product.
The first turn of the cyclic process produces four-carbon acyl group and the further turns add two carbon unit to the four-carbon acyl group. Therefore, the first turn has four carbon units and the second turn has six carbon unit in their intermediate compound.
(d)
Interpretation:
The following intermediate compound generated in the first or second cycle of the lipogenesis pathway is produced by (1) a dehydration reaction, (2) a hydrogenation reaction, or (3) a condensation reaction has to be identified.
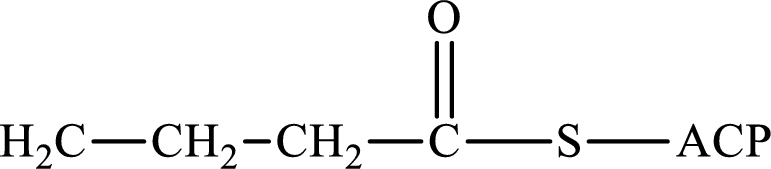
Concept introduction:
Lipogenesis is the process employed for the synthesis of fatty acid. The starting precursor for the synthesis is acetyl CoA. The enzyme employed for the process is fatty acid synthase. It is a multienzyme complex that ties the reaction responsible for the synthesis of fatty acid. The fatty acid is synthesized in two parts. In the first part, there is citrate-malate shuttle system and in the second part there is a cyclic process to synthesize saturated fatty acid.
The different reactions that are involved in the cyclic process are:

In hydrogenation reaction, a hydrogen molecule (H2) is added to an organic substance; in hydration reaction, a water molecule (H2O) is added to an unsaturated substrate; in the condensation reaction, two molecules combine to form a single product.
The first turn of the cyclic process produces four-carbon acyl group and the further turns add two carbon unit to the four-carbon acyl group. Therefore, the first turn has four carbon units and the second turn has six carbon unit in their intermediate compound.
Want to see the full answer?
Check out a sample textbook solution
Chapter 25 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- 7.5 1.93 2.05 C B A 4 3 5 The Joh. 9 7 8 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 0.86 OH 10 4 3 5 1 2 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 ppm 9 7 8 CI 4 3 5 1 2 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 2.21 4.00 1.5 2.00 2.07 1.0 ppm 2.76arrow_forwardAssign the functional group bands on the IR spectra.arrow_forwardFind the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forward
- Please help me answer these three questions. Required info should be in data table.arrow_forwardDraw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forwardTartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forward
- Including activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forwardIncluding activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forward
- Can I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,



