
Concept explainers
(a)
Interpretation:
Whether crotonate, a C4 species is a (1) hydroxy acid, (2) keto acid, (3) saturated acid, or (4) unsaturated acid has to be identified.
Concept introduction:
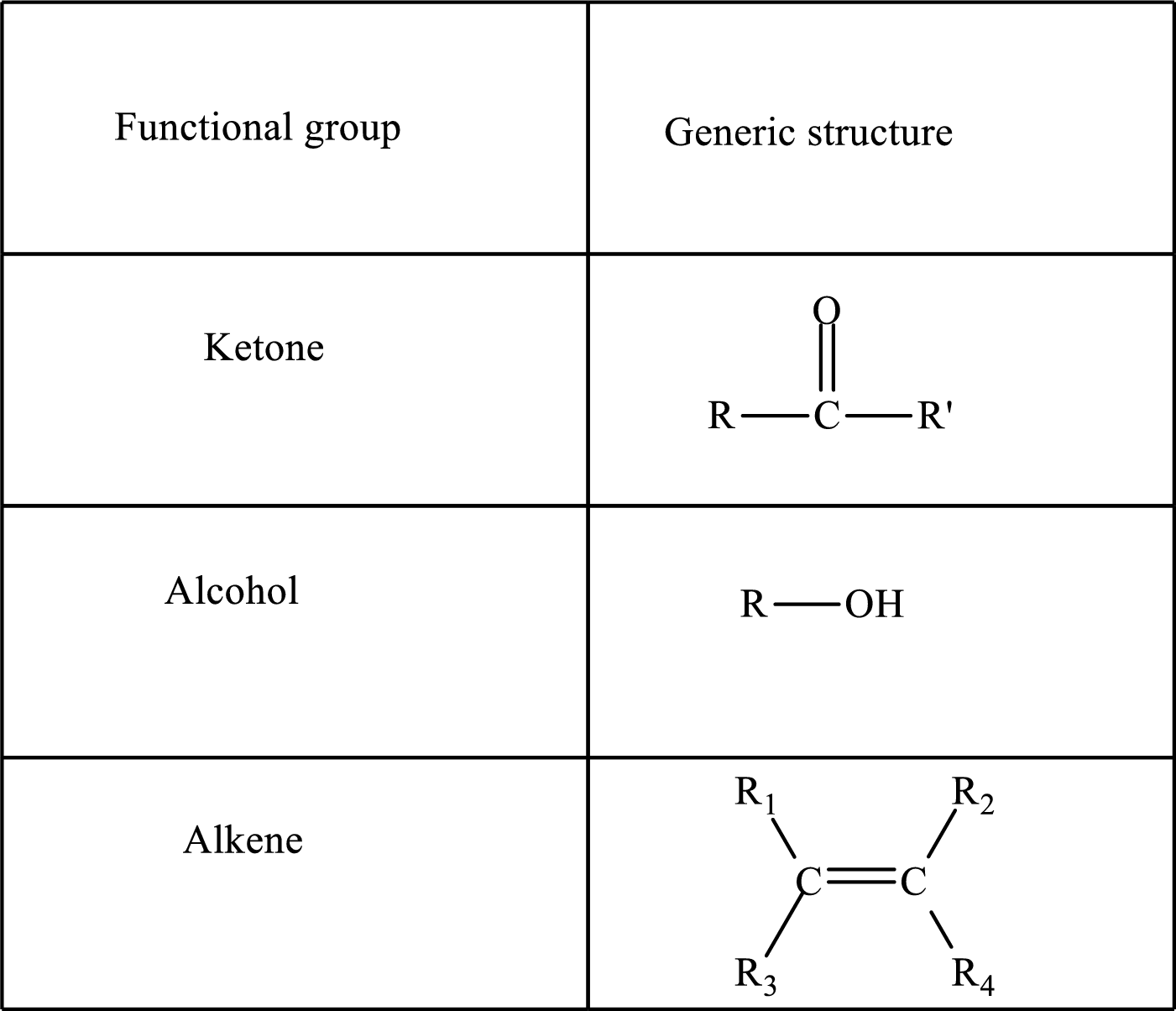
Here, R and R’ represent an alkyl group. In alkene, R1, R2, R3, and R4 can be the same or different or can be hydrogen.
Keto acid has a
A carboxylate group is formed by the removal of the acidic hydrogen from the carboxylic group. The conjugate base is formed by the removal of acidic hydrogen from the corresponding acid.
(b)
Interpretation:
Whether oxaloacetate, a C4 species is a (1) hydroxy acid, (2) keto acid, (3) saturated acid, or (4) unsaturated acid has to be identified.
Concept introduction:
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
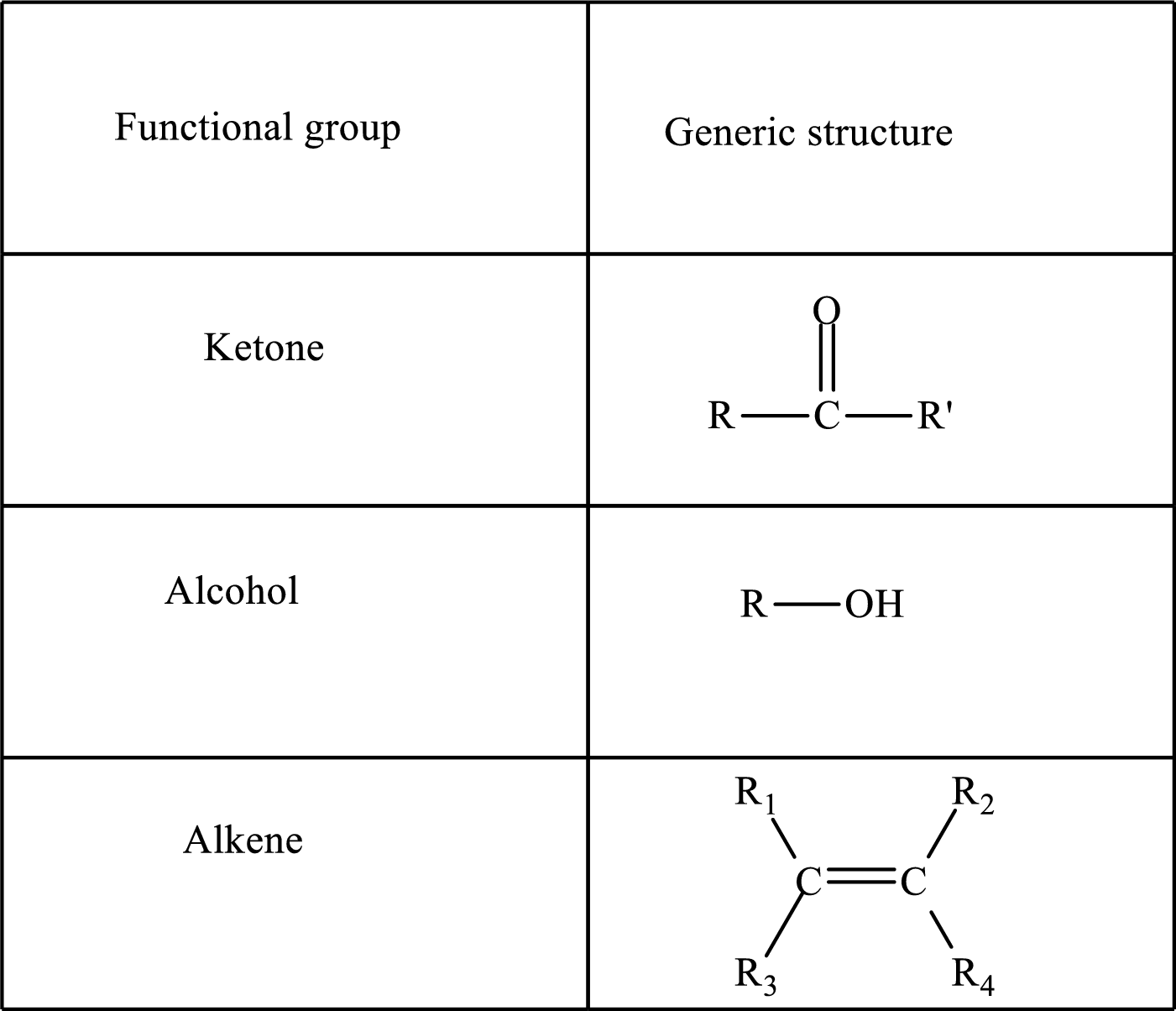
Here, R and R’ represent an alkyl group. In alkene, R1, R2, R3, and R4 can be the same or different or can be hydrogen. Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms. Alkenes have a double bond, hence; they are unsaturated compounds.
Keto acid has a ketone and a carboxylic acid (-COOH) group. Hydroxy acid has a hydroxy (-OH) group and a carboxylic acid (-COOH) group. Saturated acids contain single bonds between carbon atoms and a carboxylic group. Unsaturated acid contains a double or triple bond between carbon atoms and a carboxylic group.
A carboxylate group is formed by the removal of the acidic hydrogen from the carboxylic group. The conjugate base is formed by the removal of acidic hydrogen from the corresponding acid.
(c)
Interpretation:
Whether acetoacetate, a C4 species is a (1) hydroxy acid, (2) keto acid, (3) saturated acid, or (4) unsaturated acid has to be identified.
Concept introduction:
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
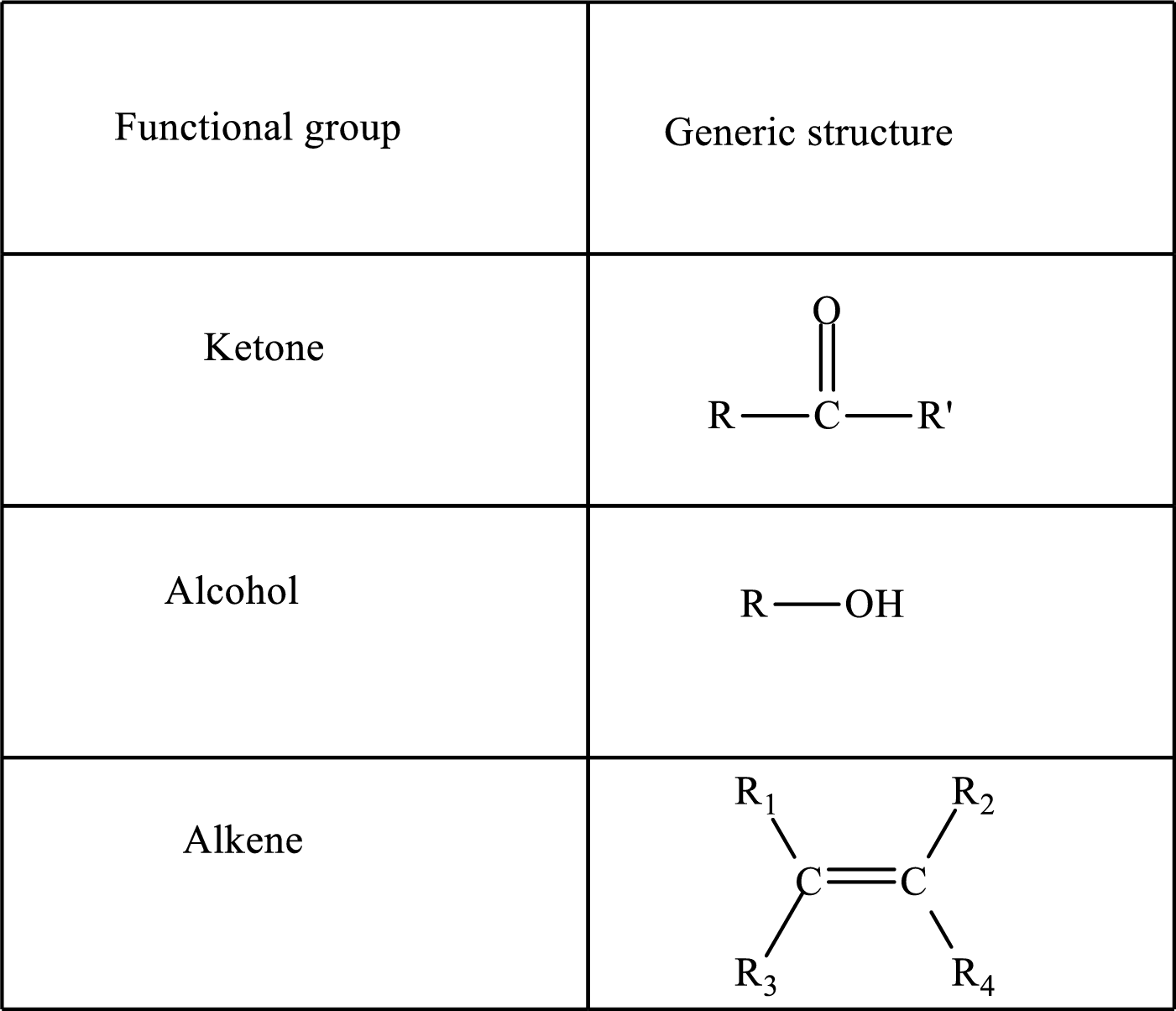
Here, R and R’ represent an alkyl group. In alkene, R1, R2, R3, and R4 can be the same or different or can be hydrogen. Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms. Alkenes have a double bond, hence; they are unsaturated compounds.
Keto acid has a ketone and a carboxylic acid (-COOH) group. Hydroxy acid has a hydroxy (-OH) group and a carboxylic acid (-COOH) group. Saturated acids contain single bonds between carbon atoms and a carboxylic group. Unsaturated acid contains a double or triple bond between carbon atoms and a carboxylic group.
A carboxylate group is formed by the removal of the acidic hydrogen from the carboxylic group. The conjugate base is formed by the removal of acidic hydrogen from the corresponding acid.
(d)
Interpretation:
Whether malate, a C4 species is a (1) hydroxy acid, (2) keto acid, (3) saturated acid, or (4) unsaturated acid has to be identified.
Concept introduction:
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
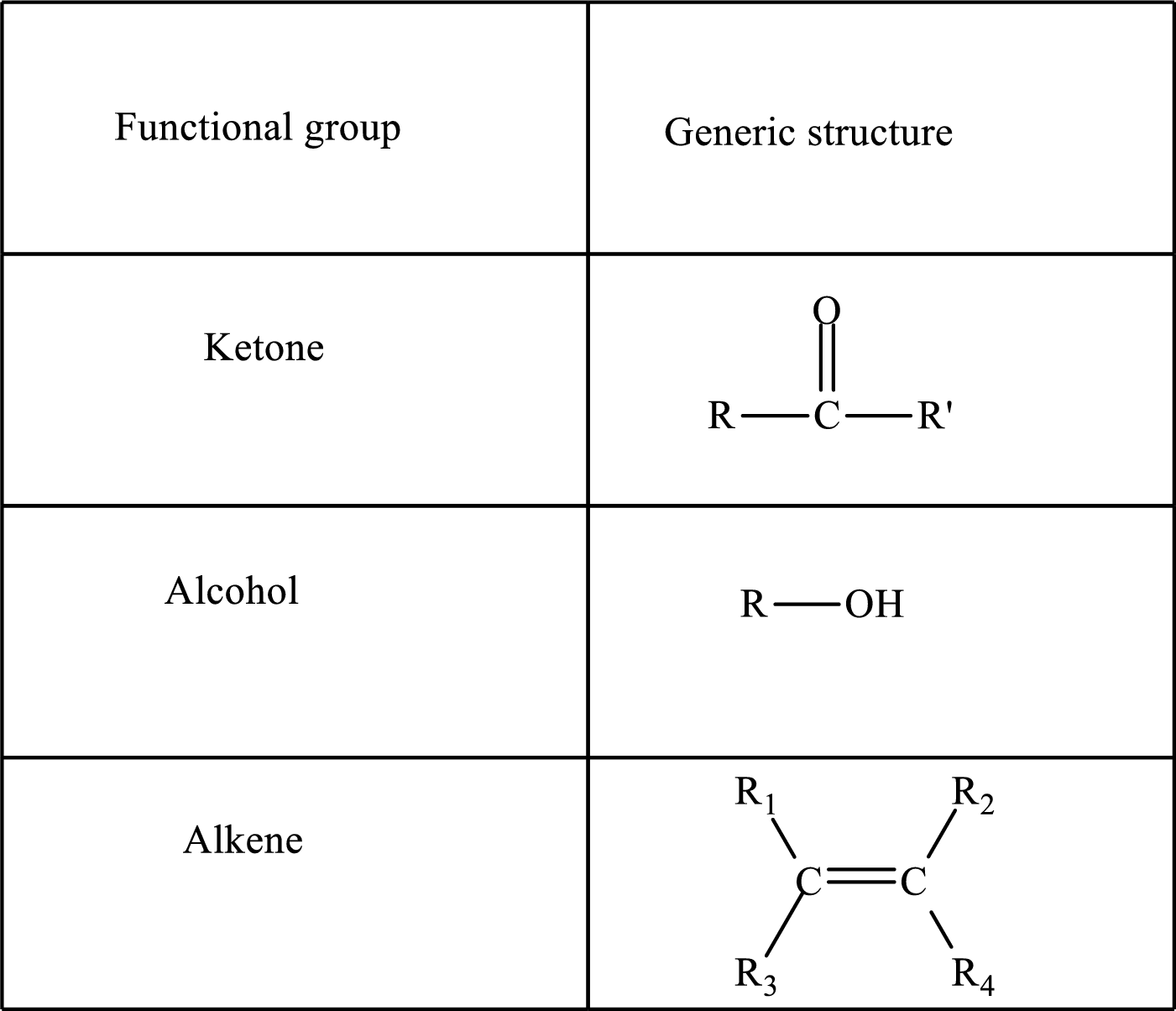
Here, R and R’ represent an alkyl group. In alkene, R1, R2, R3, and R4 can be the same or different or can be hydrogen. Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms. Alkenes have a double bond, hence; they are unsaturated compounds.
Keto acid has a ketone and a carboxylic acid (-COOH) group. Hydroxy acid has a hydroxy (-OH) group and a carboxylic acid (-COOH) group. Saturated acids contain single bonds between carbon atoms and a carboxylic group. Unsaturated acid contains a double or triple bond between carbon atoms and a carboxylic group.
A carboxylate group is formed by the removal of the acidic hydrogen from the carboxylic group. The conjugate base is formed by the removal of acidic hydrogen from the corresponding acid.
Want to see the full answer?
Check out a sample textbook solution
Chapter 25 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardGiven a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forward
- CHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the steady-state approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the limiting or determining step approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. Indicate the approximation methods for solving the rate equation.arrow_forward
- TRANSMITTANCE เบบ Please identify the one structure below that is consistent with the 'H NMR and IR spectra shown and draw its complete structure in the box below with the protons alphabetically labeled as shown in the NMR spectrum and label the IR bands, including sp³C-H and sp2C-H stretch, indicated by the arrows. D 4000 OH LOH H₂C CH3 OH H₂C OCH3 CH3 OH 3000 2000 1500 HAVENUMBERI-11 1000 LOCH3 Draw your structure below and label its equivalent protons according to the peak labeling that is used in the NMR spectrum in order to assign the peaks. Integrals indicate number of equivalent protons. Splitting patterns are: s=singlet, d=doublet, m-multiplet 8 3Hb s m 1Hd s 3Hf m 2Hcd 2Had 1He 鄙视 m 7 7 6 5 4 3 22 500 T 1 0arrow_forwardRelative Transmittance 0.995 0.99 0.985 0.98 Please draw the structure that is consistent with all the spectral data below in the box and alphabetically label the equivalent protons in the structure (Ha, Hb, Hc ....) in order to assign all the proton NMR peaks. Label the absorption bands in the IR spectrum indicated by the arrows. INFRARED SPECTRUM 1 0.975 3000 2000 Wavenumber (cm-1) 1000 Structure with assigned H peaks 1 3 180 160 140 120 100 f1 (ppm) 80 60 40 20 0 C-13 NMR note that there are 4 peaks between 120-140ppm Integral values equal the number of equivalent protons 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 fl (ppm)arrow_forwardCalculate the pH of 0.0025 M phenol.arrow_forward
- In the following reaction, the OH- acts as which of these? NO2-(aq) + H2O(l) ⇌ OH-(aq) + HNO2(aq)arrow_forwardUsing spectra attached, can the unknown be predicted? Draw the predicition. Please explain and provide steps. Molecular focrmula:C16H13ClOarrow_forwardCalculate the percent ionization for 0.0025 M phenol. Use the assumption to find [H3O+] first. K = 1.0 x 10-10arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





