
Concept explainers
(a)
Interpretation: To identify the missing substance in the electron transport chain sequences.
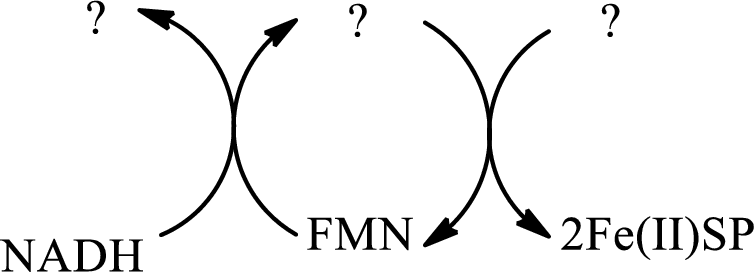
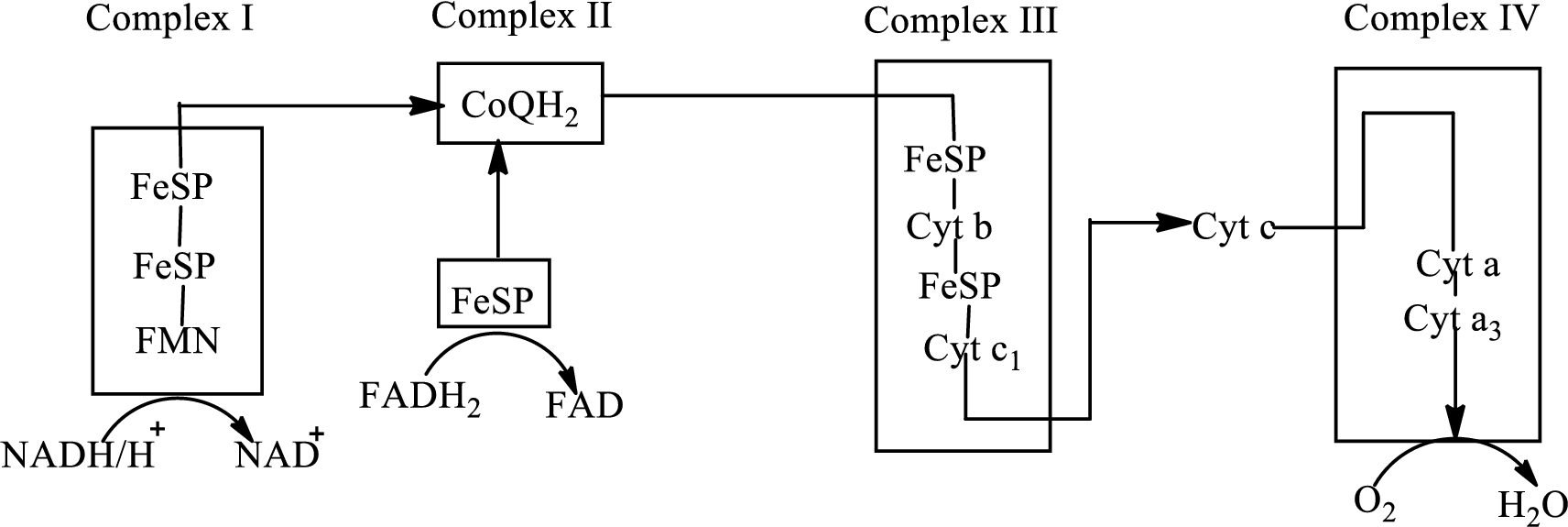
Concept introduction: Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule. There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
(b)
Interpretation: To identify the missing substance in the electron transport chain sequences.
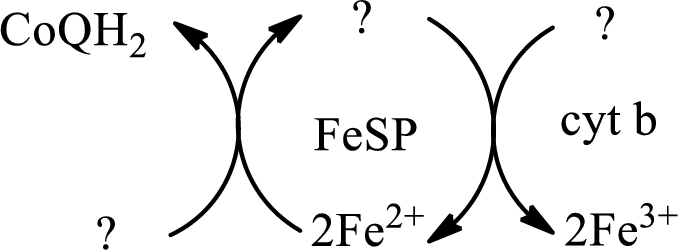
Concept introduction: Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule. There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
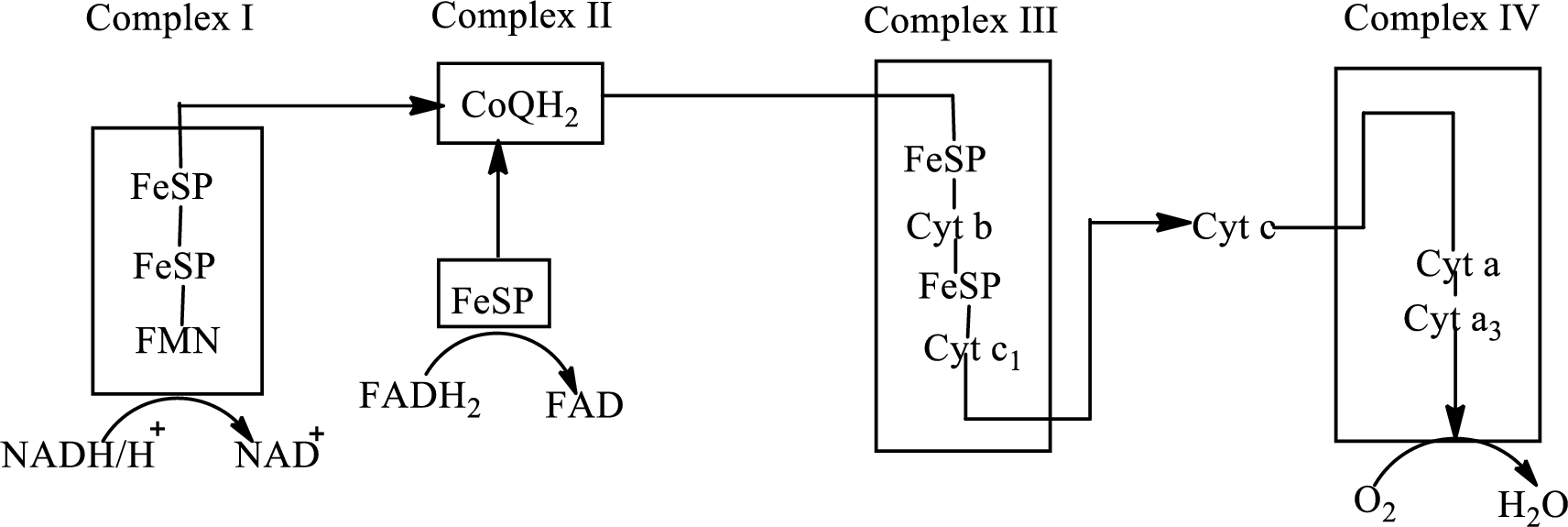
Cytochrome is a protein that consists of heme unit in its structure. The iron present in the heme unit participate in the
Trending nowThis is a popular solution!

Chapter 23 Solutions
General, Organic, and Biological Chemistry
- If a bacterium using aerobic respiration was to degrade one small protein molecule into 8 molecules of pyruvic acid, how many ATP would that cell make? Assume there is no other carbon source. Units cannot be entered in this style of question but the units of your answer should be in molecules of ATP.arrow_forwardIf a bacterium using aerobic respiration was to degrade a 30 mM solution of citric acid, how many ATP would that cell make? Assume no other carbon source is available. Units cannot be entered in this style of question but the units of your answer should be in mM of ATP.arrow_forwardHow much ATP will be produced during the following metabolic scenario: Aerobic respiration of a 5mM lipid solution that is made up of one glycerol and an 8-carbon fatty acid and 12-carbon fatty acid. Recall that when glycerol breaks down to Glyceraldehyde-3-phosphate it costs one ATP but your get an extra FADH2. Every two carbons of a fatty acid break down to one acetyl-CoA. (pathways will be provided on the exam) Units cannot be entered in this style of question but the units of your answer should be in mM of ATP.arrow_forward
- When beta-lactamase was isolated from Staphylcoccus aureus and treated with a phosphorylating agent, only the active site, serine was phosphorylated. Additionally, the serine was found to constitute 0.35% (by weight) of this beta-lactamase enzyme. Using this, calculate the molecular weight of this enzyme and estimate the number of amino acids present in the polypeptide.arrow_forwardBased on your results from the Mannitol Salt Agar (MSA) media, which of your bacteria were mannitol fermenters and which were not mannitol fermenters?arrow_forwardhelp tutor pleasearrow_forward
- Q8. A researcher wants to study the effectiveness of a pill intended to reduce stomach heartburn in pregnant women. The researcher chooses randomly 400 women to participate in this experiment for 9 months of their pregnancy period. They all need to have the same diet. The researcher designs two groups of 200 participants: One group take the real medication intended to reduce heartburn, while the other group take placebo medication. In this study what are: Independent variable: Dependent variable: Control variable: Experimental group: " Control group: If the participants do not know who is consuming the real pills and who is consuming the sugar pills. This study is It happens that 40% of the participants do not find the treatment helpful and drop out after 6 months. The researcher throws out the data from subjects that drop out. What type of bias is there in this study? If the company who makes the medication funds this research, what type of bias might exist in this research work?arrow_forwardHow do I determine the inhertiance pattern from the pedigree diagram?arrow_forwardits an open book assignemntarrow_forward
- Describe two different gene regulation mechanisms involving methylationarrow_forwardWhat is behavioral adaptarrow_forward22. Which of the following mutant proteins is expected to have a dominant negative effect when over- expressed in normal cells? a. mutant PI3-kinase that lacks the SH2 domain but retains the kinase function b. mutant Grb2 protein that cannot bind to RTK c. mutant RTK that lacks the extracellular domain d. mutant PDK that has the PH domain but lost the kinase function e. all of the abovearrow_forward
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning





