
Concept explainers
(a)
Interpretation: To indicate whether
Concept introduction: Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
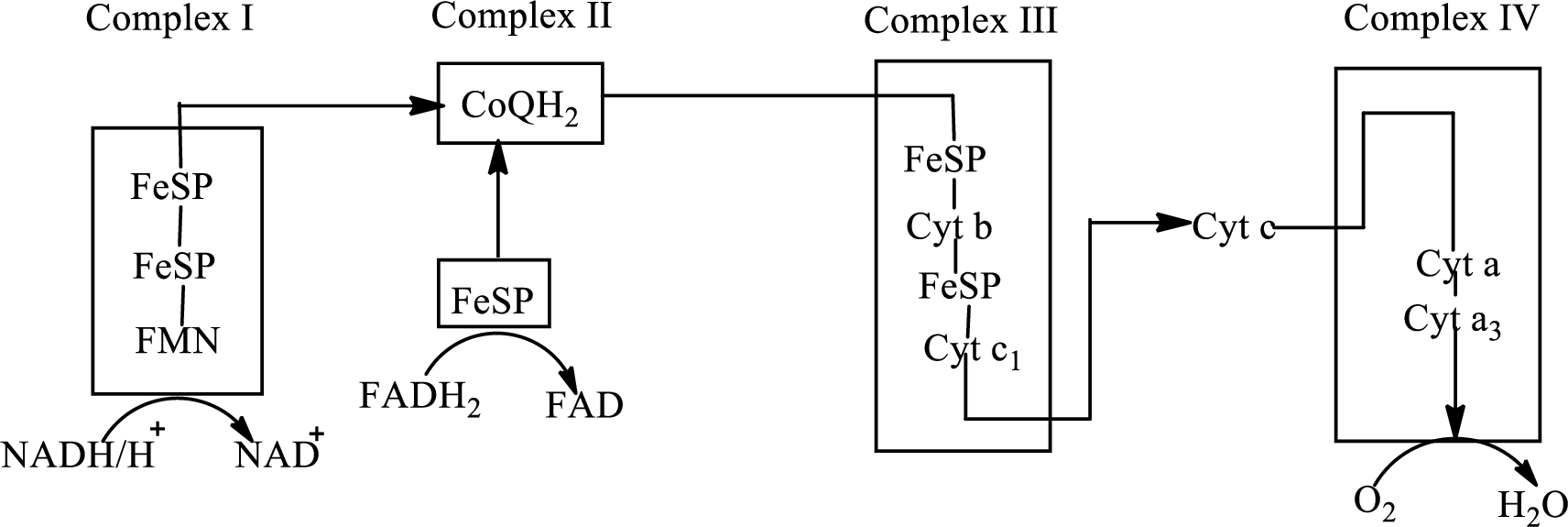
Redox
Here A is oxidized form and AH is reduced form.
(a)
Answer to Problem 23.88EP
Explanation of Solution
Here,
(b)
Interpretation: To indicate whether
Concept introduction: Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
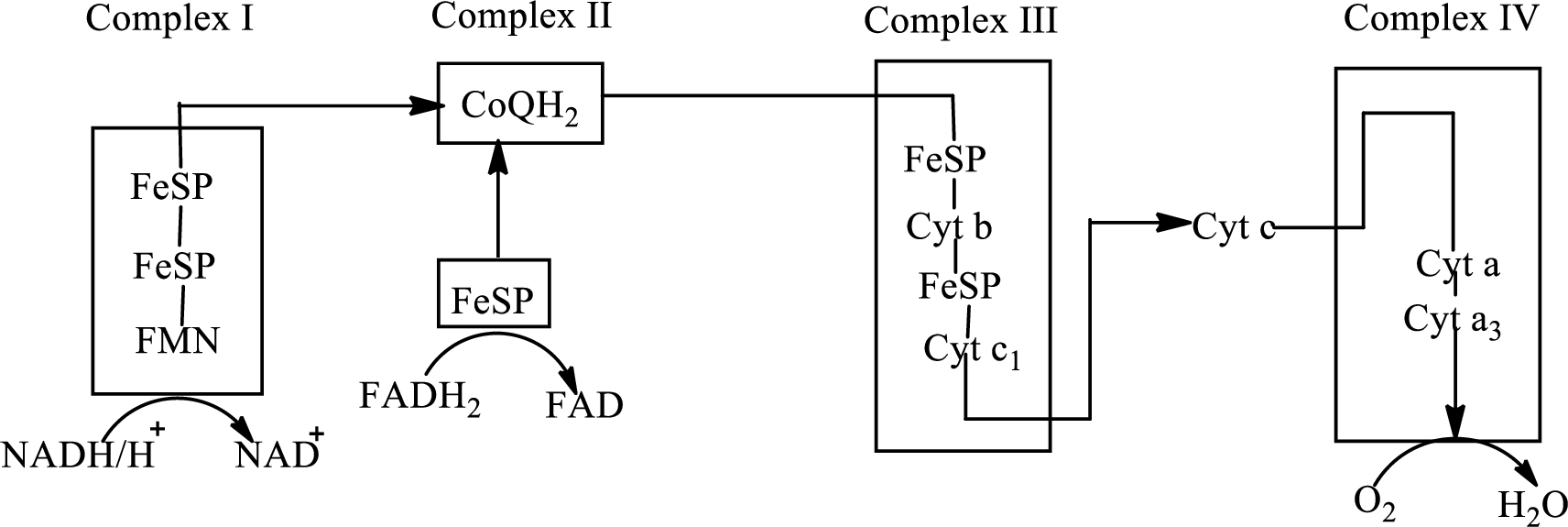
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is
Here A is oxidized form and AH is reduced form.
(b)
Answer to Problem 23.88EP
Explanation of Solution
(c)
Interpretation: To indicate whether
Concept introduction: Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
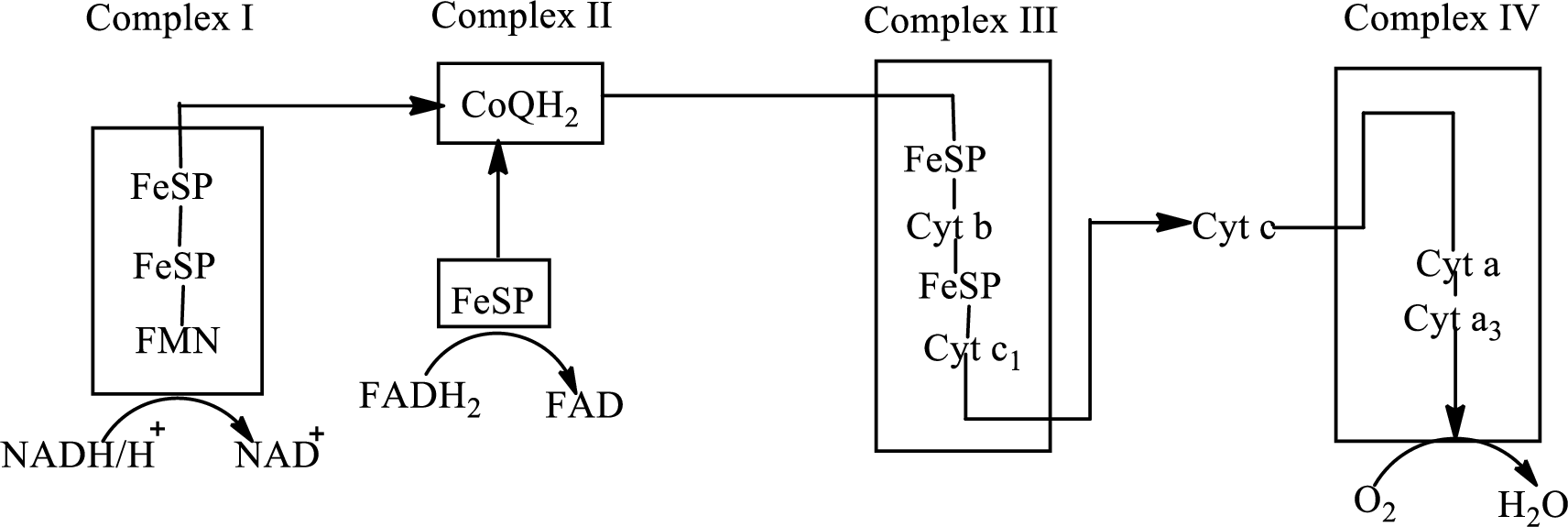
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is
Here A is oxidized form and AH is reduced form.
(c)
Answer to Problem 23.88EP
Explanation of Solution
(d)
Interpretation: To indicate whether
Concept introduction: Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
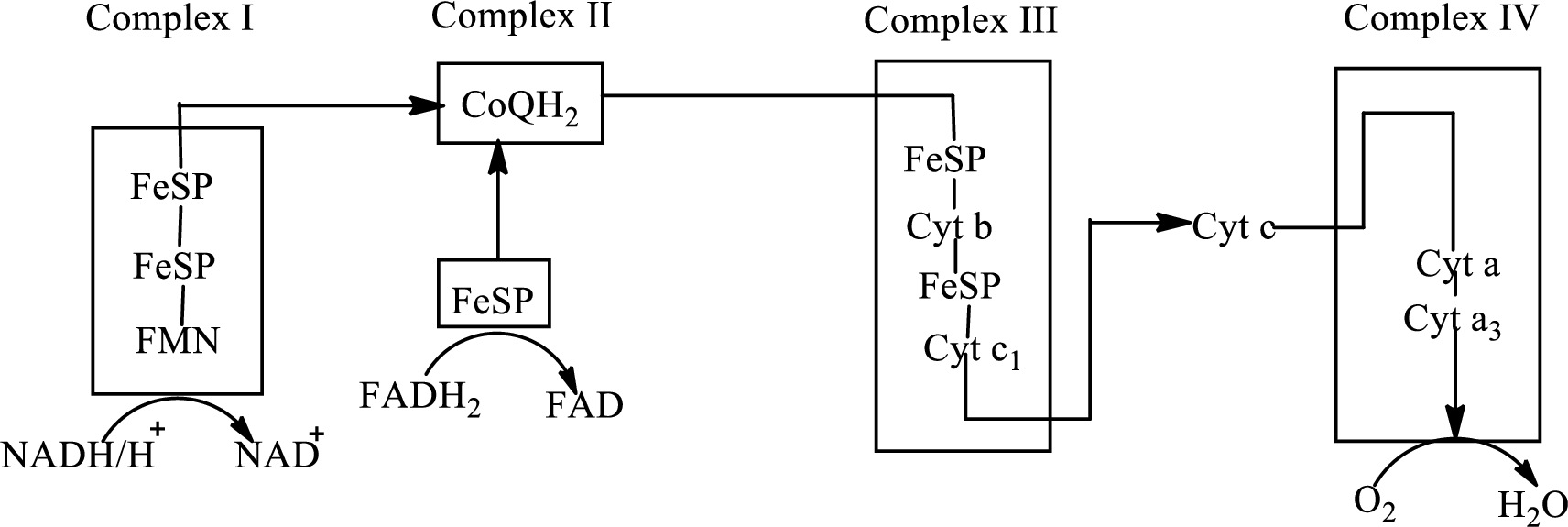
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is
Here A is oxidized form and AH is reduced form.
(d)
Answer to Problem 23.88EP
Explanation of Solution
In complex I, electrons are transferred from the
Want to see more full solutions like this?
Chapter 23 Solutions
General, Organic, and Biological Chemistry
- Predict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forward
- Predict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forwardPredict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forward
- You are trying to determine whether the following organic reaction can be done in a single synthesis step. If so, add any missing reagents or conditions in the drawing area below. If it isn't possible to do this reaction in a single synthesis step, check the box below the drawing area instead. Note for advanced students: if you have a choice of reagents to add, you should choose the least reactive and most economical reagents possible. Cl It isn't possible to do this reaction in a single synthesis step. + T OHarrow_forwardPredict the products of this organic reaction: CH3 O CH3-CH-C-O-CH2-CH2-CH3 + H₂OH+ Η ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward€ CH3-CH-C-O-CH2-CH2-CH3 + NaOH A? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Predict the products of this organic reaction: CH3 O Click anywhere to draw the first atom of your structure. No reaction ✓ Garrow_forward
- A molecule can have a temporary or permanent depending on the structure and the way the electrons can move. True Falsearrow_forwardedict the products of this organic reaction: CH3 O A CH3-CH-C-NH2 + H2O + HCI ? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Click anywhere to draw the first atom of your structure. No Reaction planation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center +arrow_forwardDraw the mechanism for the following reaction: OH A few notes: CI O • You may assume that each reagent is present in whatever amount you need to draw your mechanism. • To save you some time, one of the starting materials has been copied into the first step of the drawing area. AP Add/Remove step Cl Click and drag to start drawing a structure.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





