
Concept explainers
(a)
Interpretation: To draw the structural formula for oxaloacetic acid.
Concept introduction: There are a number of
Polyfunctional carboxylate ions act as a substrate in the metabolic pathways and can be divided into two parts depending upon the parent
Oxaloacetic acid is an unsaturated derivative of succinic acid. The structure of succinic acid is:
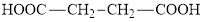
(b)
Interpretation: To draw the structural formula for oxaloacetate ion.
Concept introduction: There are a number of metabolic reaction that occur in the human body for completion of functions. During these metabolic reactions, the various metabolic intermediates are formed for the short time to complete the reactions. Polyfunctional carboxylate ions are also the metabolic intermediate formed during metabolic pathways.
Polyfunctional carboxylate ions act as a substrate in the metabolic pathways and can be divided into two parts depending upon the parent carboxylic acid. The first class consists of malate, oxaloacetate and fumarate ion which are derivative of succinic acid.
(c)
Interpretation: To draw the structural formula for citric acid.
Concept introduction: There are a number of metabolic reaction that occur in the human body for completion of functions. During these metabolic reactions, the various metabolic intermediates are formed for the short time to complete the reactions. Polyfunctional carboxylate ions are also the metabolic intermediate formed during metabolic pathways.
Polyfunctional carboxylate ions act as a substrate in the metabolic pathways and can be divided into two parts depending upon the parent carboxylic acid. The second class consists of
Citric acid is an unsaturated derivative of glutaric acid. The structure of glutaric acid is:
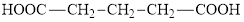
(d)
Interpretation: To draw the structural formula for citrate ion.
Concept introduction: There are a number of metabolic reaction that occur in the human body for completion of functions. During these metabolic reactions, the various metabolic intermediates are formed for the short time to complete the reactions. Polyfunctional carboxylate ions are also the metabolic intermediate formed during metabolic pathways.
Polyfunctional carboxylate ions act as a substrate in the metabolic pathways and can be divided into two parts depending upon the parent carboxylic acid. The second class consists of
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
General, Organic, and Biological Chemistry
- Skryf n kortkuns van die Egyptians pyramids vertel ñ story. Maximum 500 woordearrow_forward1.)What cross will result in half homozygous dominant offspring and half heterozygous offspring? 2.) What cross will result in all heterozygous offspring?arrow_forward1.Steroids like testosterone and estrogen are nonpolar and large (~18 carbons). Steroids diffuse through membranes without transporters. Compare and contrast the remaining substances and circle the three substances that can diffuse through a membrane the fastest, without a transporter. Put a square around the other substance that can also diffuse through a membrane (1000x slower but also without a transporter). Molecule Steroid H+ CO₂ Glucose (C6H12O6) H₂O Na+ N₂ Size (Small/Big) Big Nonpolar/Polar/ Nonpolar lonizedarrow_forward
- what are the answer from the bookarrow_forwardwhat is lung cancer why plants removes liquid water intead water vapoursarrow_forward*Example 2: Tracing the path of an autosomal dominant trait Trait: Neurofibromatosis Forms of the trait: The dominant form is neurofibromatosis, caused by the production of an abnormal form of the protein neurofibromin. Affected individuals show spots of abnormal skin pigmentation and non-cancerous tumors that can interfere with the nervous system and cause blindness. Some tumors can convert to a cancerous form. i The recessive form is a normal protein - in other words, no neurofibromatosis.moovi A typical pedigree for a family that carries neurofibromatosis is shown below. Note that carriers are not indicated with half-colored shapes in this chart. Use the letter "N" to indicate the dominant neurofibromatosis allele, and the letter "n" for the normal allele. Nn nn nn 2 nn Nn A 3 N-arrow_forward
- I want to be a super nutrition guy what u guys like recommend mearrow_forwardPlease finish the chart at the bottom. Some of the answers have been filled in.arrow_forward9. Aerobic respiration of one lipid molecule. The lipid is composed of one glycerol molecule connected to two fatty acid tails. One fatty acid is 12 carbons long and the other fatty acid is 18 carbons long in the figure below. Use the information below to determine how much ATP will be produced from the glycerol part of the lipid. Then, in part B, determine how much ATP is produced from the 2 fatty acids of the lipid. Finally put the NADH and ATP yields together from the glycerol and fatty acids (part A and B) to determine your total number of ATP produced per lipid. Assume no other carbon source is available. 18 carbons fatty acids 12 carbons 9 glycerol A. Glycerol is broken down to glyceraldehyde 3-phosphate, a glycolysis intermediate via the following pathway shown in the figure below. Notice this process costs one ATP but generates one FADH2. Continue generating ATP with glyceraldehyde-3-phosphate using the standard pathway and aerobic respiration. glycerol glycerol-3- phosphate…arrow_forward
- Normal dive (for diving humans) normal breathing dive normal breathing Oz level CO2 level urgent need to breathe Oz blackout zone high CO2 triggers breathing 6. This diagram shows rates of oxygen depletion and carbon dioxide accumulation in the blood in relation to the levels needed to maintain consciousness and trigger the urgent need to breathe in diving humans. How might the location and slope of the O₂ line differ for diving marine mammals such as whales and dolphins? • How might the location and slope of the CO₂ line differ for diving marine mammals such as whales and dolphins? • • Draw in predicted lines for O2 and CO2, based on your reasoning above. How might the location of the Urgent Need to Breathe line and the O2 Blackout Zone line differ for diving marine mammals? What physiological mechanisms account for each of these differences, resulting in the ability of marine mammals to stay submerged for long periods of time?arrow_forwardforaging/diet type teeth tongue stomach intestines cecum Insectivory numerous, spiky, incisors procumbentExample: moleExample: shrew -- simple short mostly lacking Myrmecophagy absent or reduced in numbers, peg-likeExample: tamandua anteater extremely long simple, often roughened short small or lacking Terrestrial carnivory sharp incisors; long, conical canines; often carnassial cheek teeth; may have crushing molarsExample: dog -- simple short small Aquatic carnivory homodont, spiky, numerousExample: common dolphin -- simple or multichambered (cetaceans only) variable small or absent Sanguinivory very sharp upper incisors; reduced cheek teethExample: vampire bat grooved tubular, highly extensible long small or lacking Herbivory (except nectivores) incisors robust or absent; canines reduced or absent; diastema; cheek teeth enlarged with complex occlusal surfacesExample: beaver -- simple (hindgut fermenters) or multichambered (ruminants) long large Filter feeding none…arrow_forward3. Shown below is the dental formula and digestive tract anatomy of three mammalian species (A, B, and C). What kind of diet would you expect each species to have? Support your answers with what you can infer from the dental formula and what you can see in the diagram. Broadly speaking, what accounts for the differences? Species A 3/3, 1/1, 4/4, 3/3 པར『ན་ cm 30 Species B 4/3, 1/1, 2/2, 4/4 cm 10 Species C 0/4, 0/0,3/3, 3/3 020arrow_forward
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage LearningEssentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage





