
Concept explainers
(a)
Interpretation: To determine the number of NADH molecules formed during one turn of the citric acid cycle.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
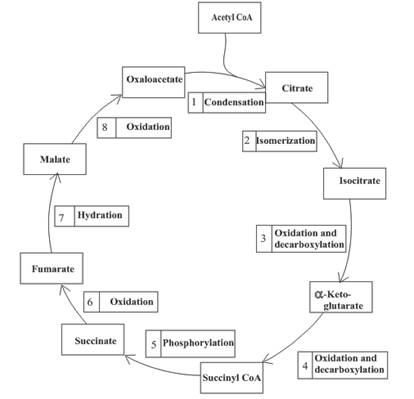
(a)
Answer to Problem 23.70EP
Three molecules of NADH are formed in step 3, 4 and 8 of the citric acid cycle.
Explanation of Solution
Step 3 is the first step where both the oxidation and decarboxylation occurs. Step 3 involves the oxidation of isocitrate and formation of CO2. In this step, firstly isocitrate is oxidized by
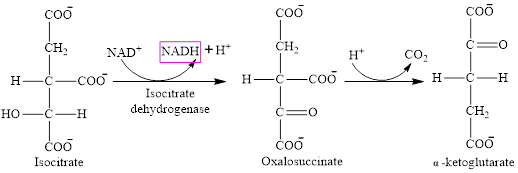
Step 4 involves the oxidation of

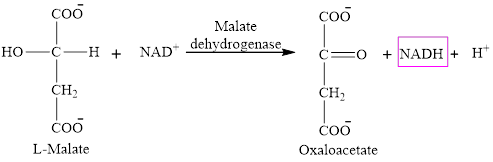
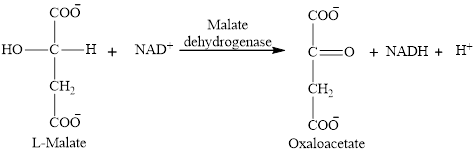
Step 8 is an oxidation reaction and the last step of the citric acid cycle. In step 8,
The reaction of step 8 is:
Hence, three molecules of NADH are formed in the citric acid cycle.
(b)
Interpretation: To determine the number of GTP molecules formed during one turn of the citric acid cycle.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
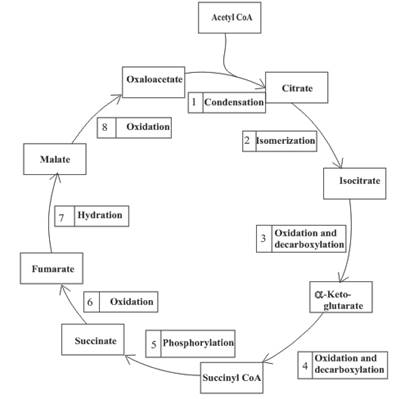
(b)
Answer to Problem 23.70EP
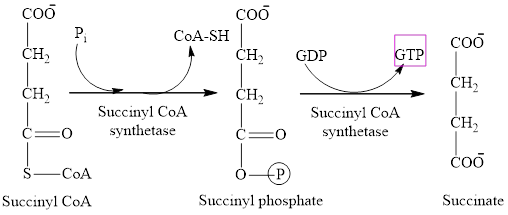
One molecule of GTP is formed in step 5 of the citric acid cycle.
Explanation of Solution
Step 5 involves the thioester bond cleavage in
(c)
Interpretation: To determine the number of time decarboxylation reactions occur during one turn of the citric acid cycle.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
(c)
Answer to Problem 23.70EP
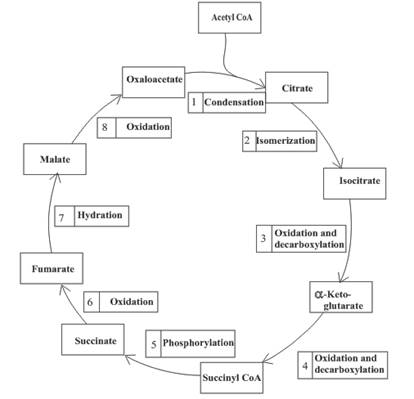
Decarboxylation occurs twice in the citric acid cycle in step 3 and 4.
Explanation of Solution
Step 3 is the first step where both the oxidation and decarboxylation occurs. Step 3 involves the oxidation of isocitrate and formation of CO2. In this step, firstly isocitrate is oxidized by
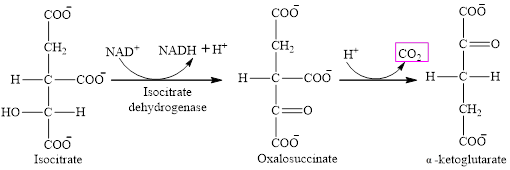
The final product is
Step 4 involves the oxidation of

The final product is
(d)
Interpretation: To determine the number of time oxidation-reduction reaction occur during one turn of the citric acid cycle.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
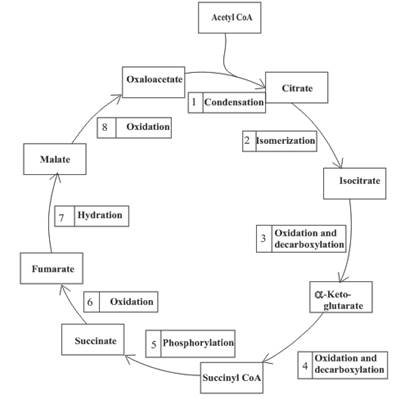
An overview of the citric acid cycle is as follows:
(d)
Answer to Problem 23.70EP
The oxidation-reduction reaction occurs four times in the citric acid cycle in step 3, 4, 6 and 8.
Explanation of Solution
Step 3 is the first step where both the oxidation and decarboxylation occurs. Step 3 involves the oxidation of isocitrate and formation of CO2. In this step, firstly isocitrate is oxidized by
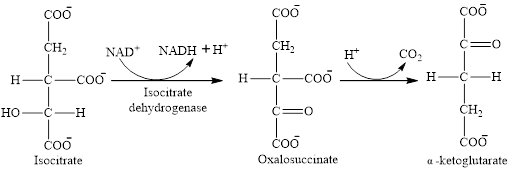
Step 4 involves the oxidation of

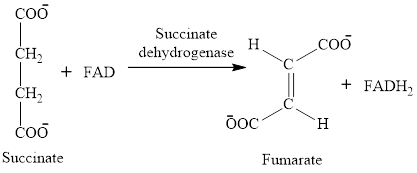
In step 6, oxidation of succinate occurs to form fumarate. The enzyme involved in this step of the citric acid cycle is succinate dehydrogenase. FAD is the oxidizing agent in this step. This reaction takes place in the inner mitochondrial membrane. The reaction of step 6 is:
Step 8 is an oxidation reaction and the last step of the citric acid cycle. In step 8,
The reaction of step 8 is:
Want to see more full solutions like this?
Chapter 23 Solutions
General, Organic, and Biological Chemistry
- Predict the products of this organic reaction: O N IN A N + H2O + HCI ? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. 田 C + Explanation Check Click and drag to start drawing a structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward6. For each of the following, fill in the synthesis arrows with reagents and show the intermediates. You DO NOT need to use the same number of arrows that are shown (you may use more or less), but the product must be formed from the reactant. Then write the mechanism of one step in the synthesis (you can choose which step to write the mechanism for), including all reagents required, clearly labeling the nucleophile and electrophile for each step, and using curved arrows to show the steps in the mechanism. a. b. OHarrow_forwardDraw the productsarrow_forward
- Draw the correct productsarrow_forwardE Organic Chemistry Maxwell Draw the correct products, in either order, for the ozonolysis reaction: 1) O3, CH2Cl2, -78 °C Product 1 + Product 2 2) Zn, HOAc Draw product 1. Select Draw Templates More C H O presented by M Draw product 2. Erase Select Draw Templates M / # # carrow_forward✓ edict the products of this organic reaction: ---- ။ A CH3–C−NH–CH2–C−CH3 + KOH ? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibiliarrow_forward
- Predict the product of this organic reaction: A HO-C-CH3 + CH3NH2 P+ H2O Specifically, in the drawing area below draw the condensed structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. marrow_forwardH 1) OsO4, pyridine 2) Na2SO3 or NaHSO3 in H₂O 2 productsarrow_forward● Biological Macromolecules Naming and drawing cyclic monosaccharides Your answer is incorrect. • Row 1: Your answer is incorrect. Row 3: Your answer is incorrect. • Row 4: Your answer is incorrect. Try again... 0/5 Give the complete common name, including anomer and stereochemistry labels, of the following molecules. You will find helpful information in the ALEKS resource. CH2OH OH OH H H I H OH OH H] H CH2OH H OH ẞ-L-sorbose HOCH2 OH OH H HOCH2 H OH OH H OH H H CH2OH OH H H OH H I- H OH H OH Explanation Recheck W E R % 25 α B Y X & 5 D F G H McGraw Hill LLC. All Rights Reserved. Terms of Use | Pr Parrow_forward
- What is the missing reactant in this organic reaction? + R -A HO IN + H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardStuc X ctclix ALE X A ALE אן A ALEX Lab (195 X Nut x M Inb x NU X NUT X Unt x + → C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IQ1g8NUi-mObKa_ZLx2twjEhK7mVG6PulJI006NcKTV37JxMpZuyrVCdQolLAKqp_7U3r1GUD3... New Chrome available: Naomi Question 26 of 39 (4 points) | Question Attempt: 1 of Unlimited Give the IUPAC name. 2,3-dimethylhexane Part: 1/2 Part 2 of 2 Draw the skeletal structure of a constitutional isomer of the alkane above that contains a different number of carbons in its longest chain. Skip Part Check Click and drag to start drawing a structure. 3 Finance headline Q Search mwa Harvard Intensifi... X Save For Later 00 dlo HB Submit Assignment 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility a 9:11 PM 4/22/2025arrow_forwardPredict the product of this organic reaction: + NH2 HO A P+ H2O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Click and drag to start drawing a structure. ✓arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning




