
(a)
Interpretation:
The structure of 1-propanol needs to be drawn.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix,
Answer to Problem 11SSC

Explanation of Solution
The given name of the compound is 1-propanol.
The root name is “prop” thus, there are 3 carbon atoms in the main chain. Suffix −ol represents the presence of hydroxyl group or −OH group and prefix 1- represents the position of hydroxyl group.
Thus, the structure of compound will be:

(b)
Interpretation:
The structure of 1, 3-cyclopentanediol needs to be drawn.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. For example, alcohol group will have suffix −ol, aldehyde will have suffix −al. If there is no functional group the suffix will be −ane. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain. Also, in case of cyclic molecules prefix cyclo- is used.
Answer to Problem 11SSC

Explanation of Solution
The given name of the compound is 1, 3-cyclopentanediol.
The root name is “pent” thus, there are 5carbon atoms in the main chain. Suffix −
Thus, the structure of compound will be:

(c)
Interpretation:
The structure of propyl ether needs to be drawn.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. For example, alcohol group will have suffix −ol, aldehyde will have suffix −al. If there is no functional group the suffix will be −ane. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
Answer to Problem 11SSC

Explanation of Solution
The given name of the compound is propyl ether.
The root name is “prop” thus, there are 3 carbon atoms. But there is ether group present which shows the oxygen atom between two propyl groups.
Thus, the structure of compound will be:

(d)
Interpretation:
The structure of 1, 2-propanediamine needs to be drawn.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. For example, alcohol group will have suffix −ol, aldehyde will have suffix −al. If there is no functional group the suffix will be −ane. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
Answer to Problem 11SSC
Explanation of Solution
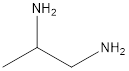
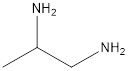
The given name of the compound is 1, 2-propanediamine.
The root name is “prop” thus, there are 3 carbon atoms. Suffix −diamine represents presence of two amino groups and prefix 1, 2- represents the position of two amino groups.
Thus, the structure of compound will be:
Chapter 22 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
College Physics: A Strategic Approach (3rd Edition)
Anatomy & Physiology (6th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Applications and Investigations in Earth Science (9th Edition)
Human Physiology: An Integrated Approach (8th Edition)
- 2. Please fill in missing reactants, reagents, reaction conditions, or products in the provided blank boxes OMe ...-CF2-CF2-CF2-CF2-CF2-...arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardI don't understand what to put for final step. Does that just mean termination? And would a radical form when I add bromine to ch2 between the rings?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





