
a.
Interpretation:
The type of organic reaction that converts
Concept introduction:
Structural isomers have same number of atoms of element in the compounds but the position of groups is different. An
a.
Explanation of Solution
Addition with hydrogen gas and platinum results in the reduction of double bond and formation of alkane from alkene takes place.
The general reaction is represented as follows:
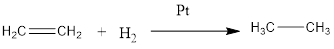
Thus, it is an addition reaction.
b.
Interpretation:
The type of organic reaction that converts alkyl halide to alcohol needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix,
b.
Explanation of Solution
An alkyl halide can be converted to alcohol after addition of water or hydroxide. This is a nucleophillic substitution reaction.
The general reaction is represented as follows:
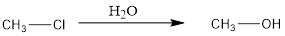
This is an addition reaction. It can also known as hydrolysis reaction as water is added to the alkyl halide. Also, there is substitution of −OH group takes place at the place of Cl thus, it is a type of substitution reaction as well.
c.
Interpretation:
The type of organic reaction that converts alkyl halide to alkene needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
c.
Explanation of Solution
Alkyl halide form alkene after treatment with strong base. The reaction is 1, 2-eliminatio reaction. The bases generally used are NaOH or KOH.
The general reaction is represented as follows:

The above reaction is an elimination reaction.
d.
Interpretation:
The type of organic reaction that converts alcohol to alkyl halide needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
d.
Explanation of Solution
An alcohol can be converted to alkyl halide after reaction with H-X where X is a halide group.
The general reaction is represented as follows:
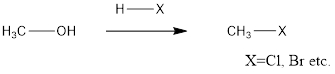
The above reaction is a substitution reaction.
e.
Interpretation:
The type of organic reaction that converts alkyl halide to alkene needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
e.
Explanation of Solution
Alkyl halide form alkene after treatment with strong base. The reaction is 1, 2-eliminatio reaction. The bases generally used are NaOH or KOH.
The general reaction is represented as follows:

This is an elimination reaction.
f.
Interpretation:
The type of organic reaction that converts alkene to alcohol needs to be determined.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
f.
Explanation of Solution
The conversion of alkene to alcohol takes place after reaction with steam at definite temperature and pressure in the presence of phosphoric acid catalyst.
The general reaction is represented as follows:
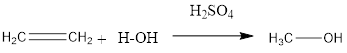
This is an acid catalyzed hydration reaction.
Chapter 22 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Human Anatomy & Physiology (2nd Edition)
Human Physiology: An Integrated Approach (8th Edition)
Organic Chemistry (8th Edition)
Campbell Biology (11th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Campbell Biology in Focus (2nd Edition)
- Draw a tetramer of this alternating copolymer.arrow_forwardH I T H HH H -H C. H- Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H CIH H H H HHHH H H 0 ·H H– 冊 CH CHI HH C- H- H H- H H A. H H C H H- -H HH H B. H- -H D. H H H H • H -H E. -H H H HICH T HHH F. H-arrow_forwardPolylactic acid (shown below) is a biodegradable polymer used for food packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forward
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forwardPoly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forward
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





