
Interpretation:
The reason for ethanoic acid to be soluble in water but palmitic acid to be insoluble in water needs to be explained.
Concept introduction:
An organic compound can be soluble in water if it shows effective hydrogen bond formation with water molecules. The stronger is the interaction with water, more soluble is the compound.
Explanation of Solution
The structure of ethanoic acid (acetic acid) is represented as follows:
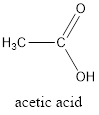
The hydrogen bond formation is represented as follows:
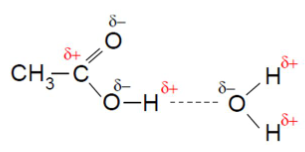
Also, it can undergo dimerization by forming hydrogen bond with it self.
The structure of palmitic acid is as follows:
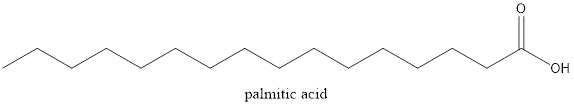
It can form hydrogen bond with water molecule but, due to long carbon chain the hydrogen bonding is not dominant intermolecular force.
If the carbon chain of an alcohol increases, its solubility in water decreases. Due to large number of carbon atoms in the carbon chain there is greater magnitude of dispersion forces between the chains. Therefore, more important intermolecular force is dispersion force in case of long carbon atom chain. This results in decrease in the solubility of higher alcohols in water.
Thus, ethanoic acid is water soluble but long chain carboxylic acids like palmitic acid is water insoluble.
Chapter 22 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Applications and Investigations in Earth Science (9th Edition)
Cosmic Perspective Fundamentals
College Physics: A Strategic Approach (3rd Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Campbell Biology in Focus (2nd Edition)
Anatomy & Physiology (6th Edition)
- A partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forwardProblem 38 can u explain and solve thanks april 24arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





