
(a)
Interpretation:
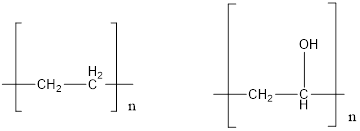
The
Concept introduction:
The monomer units combined together to form polymer in the
Explanation of Solution
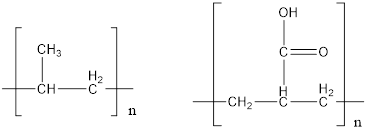
The given polymers are as follows:
The second polymer has an ionizable hydrogen ion which causes polarity in the molecule. Since, water is a polar solvent, it can dissolve more polar molecule. Therefore, second polymer being polar in nature can dissolved in water more strongly than first polymer which does not have any polarity.
(b)
Interpretation:
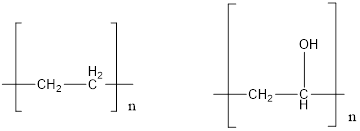
The polymer which is more soluble in water needs to be predicted.
Concept introduction:
The monomer units combined together to form polymer in the polymerization reaction. There are two types: addition and condensation polymerization.
Explanation of Solution
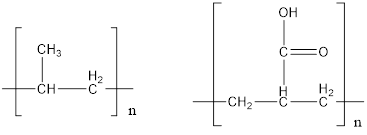
The given polymers are as follows:
The second polymer has an ionizable hydrogen ion which causes polarity in the molecule. Since, water is a polar solvent, it can dissolve more polar molecule. Therefore, second polymer being polar in nature can dissolved in water more strongly than first polymer which does not have any polarity.
Chapter 22 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Human Physiology: An Integrated Approach (8th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Campbell Biology (11th Edition)
Microbiology: An Introduction
Chemistry: Structure and Properties (2nd Edition)
Applications and Investigations in Earth Science (9th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





