
Concept explainers
a.
Interpretation:
The name of the structural isomer needs to be determined which can be formed by changing the position of one or more halogen atom in the 2-chrolopentane.
Concept introduction:
Structural isomers have same number of atoms of element in the compounds but the position of groups is different. An
a.
Explanation of Solution
The structure of 2-chloropentane is represented as follows:
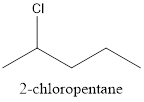
Now, the isomer can be formed by changing the position of chlorine atom as follows:
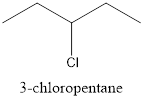
This is known as 3-chloropentane.
b.
Interpretation:
The name of the structural isomer needs to be determined which can be formed by changing the position of one or more halogen atom in the 1, 1-difluoropropane.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix,
b.
Explanation of Solution
The structure of 1, 1-difluoropropane is represented as follows:
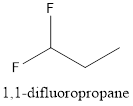
Now, the isomer can be formed by changing the position of fluorine atom as follows:
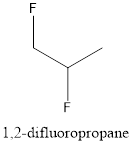
This is known as 1, 2-difluoropropane.
c.
Interpretation:
The name of the structural isomer needs to be determined which can be formed by changing the position of one or more halogen atom in the 1, 3-dibromocyclopentane.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
c.
Explanation of Solution
The structure of 1, 3-dibromocyclopentane is represented as follows:
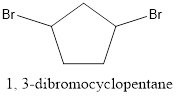
Now, the isomer can be formed by changing the position of bromine atom as follows:
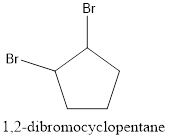
This is known as 1, 2-dibromocyclopentane.
d.
Interpretation:
The name of the structural isomer needs to be determined which can be formed by changing the position of one or more halogen atom in the 1-bromo-2-chloroethane.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
d.
Explanation of Solution
The structure of 1-bromo-2-chloroethane is represented as follows:
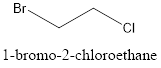
Now, the isomer can be formed by changing the position of bromine or chlorine atom as follows:
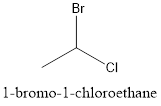
This is known as 1-bromo-1-chloroethane.
Chapter 22 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Introductory Chemistry (6th Edition)
Microbiology: An Introduction
Anatomy & Physiology (6th Edition)
Campbell Biology (11th Edition)
- A partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forwardProblem 38 can u explain and solve thanks april 24arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





