
(a)
Interpretation:
The two uses of polypropylene need to be listed.
Concept introduction:
The monomer units combined together to form
In addition polymerization, two monomer units add together without release of any side product. In condensation polymerization, addition of two monomer units takes place with release of side product.
Explanation of Solution
The given polymer is as follows:
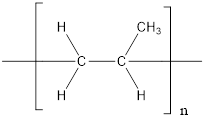
The formation of polypropylene is represented as follows:
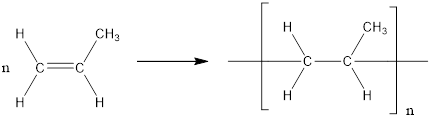
From the above reaction, the above polymer is formed from propylene via addition polymerization.
The two uses are as follows:
It is a thermoplastic polymer and it is used in packaging products. It is also used as plastic parts for number of industries and special devices in textiles industries.
(b)
Interpretation:
The two uses of polyurethane need to be listed.
Concept introduction:
The monomer units combined together to form polymer in the polymerization reaction. There are two types: addition and condensation polymerization.
In addition polymerization, two monomer units add together without release of any side product. In condensation polymerization, addition of two monomer units takes place with release of side product.
Explanation of Solution
The given polymer is as follows:
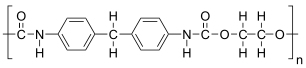
The formation of polymer is represented as follows:
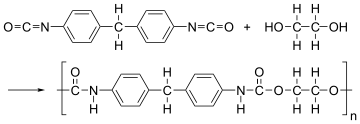
From the above reaction, it is formed by condensation polymerization of polyol (alcohol containing more than 2 hydroxyl groups which are reactive) and diisocyanate in the presence of a catalyst.
The two uses of the polymer are as follows:
Polyurethane is used in the formation of durable elastomeric tires and wheels as well as hard plastic parts in automobile industries. They are also used in rigid foam insulation panels.
(c)
Interpretation:
The two uses of polytetrafluoroethylene need to be listed.
Concept introduction:
The monomer units combined together to form polymer in the polymerization reaction. There are two types: addition and condensation polymerization.
In addition polymerization, two monomer units add together without release of any side product. In condensation polymerization, addition of two monomer units takes place with release of side product.
Explanation of Solution
The structure of polytetrafluoroethylene polymer is as follows:
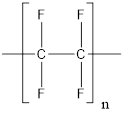
From the above structure, it can be seen that the repeating units are:
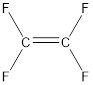
Thus, the monomer for the given polymer is tetrafluoroethylene.
The two uses of the polymer are as follows:
Polytetrafluoroethylene is used as non-stick coating on pans and pressure cooker. It is also used in pipework and containers for corrosive and reactive chemicals.
(d)
Interpretation:
The two uses of polyvinyl chloride need to be listed.
Concept introduction:
The monomer units combined together to form polymer in the polymerization reaction. There are two types: addition and condensation polymerization.
In addition polymerization, two monomer units add together without release of any side product. In condensation polymerization, addition of two monomer units takes place with release of side product.
Explanation of Solution
The given polymer is polyvinyl chloride. Here, the monomer is vinyl chloride. The formation of polymer is represented as follows:
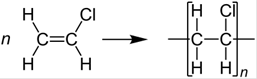
The two uses of the polymer are as follows:
The polymer is used in a variety of application in constriction, electronics, health care and automobile sectors. For example, it is used in the manufacturing of blood bags in health care sectors. It is also used as wire and cable insulation in electronics sector.
Chapter 22 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Microbiology with Diseases by Body System (5th Edition)
Campbell Biology (11th Edition)
Campbell Biology in Focus (2nd Edition)
Biology: Life on Earth (11th Edition)
Microbiology: An Introduction
- A partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forwardProblem 38 can u explain and solve thanks april 24arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





