
Concept explainers
a.
Interpretation:
The structure of chlorobenzene needs to be drawn.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix,
a.
Explanation of Solution
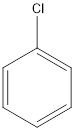
The given compound is chlorobenzene.
As per the name, there is a benzene ring with chlorine group attached to it.
The structure will be:
b.
Interpretation:
The structure of 1-bromo-4-chlorohexane needs to be drawn.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
b.
Explanation of Solution
The given compound is 1-bromo-4-chlorohexane.
From the name, there are 6 carbon atoms in the chain. Also, there is 1 bromo group at 1st position and 1 chloro group at 4th position.
Thus, the structure of compound will be:
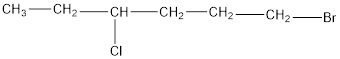
c.
Interpretation:
The structure of 1, 2-difluoro-3-iodocyclohexane needs to be drawn.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
c.
Explanation of Solution
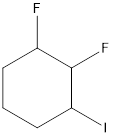
The given compound is 1, 2-difluoro-3-iodocyclohexane.
From the name it can be seen that there are 6 carbon atoms in the ring. Also, there is 1 I at 3rd position and 2 F groups at 1 and 2 positions.
The structure of the compound will be:
d.
Interpretation:
The structure of 1, 3-dibromobenzene needs to be drawn.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
d.
Explanation of Solution
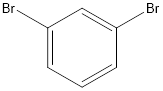
The name of the compound is 1, 3-dibromobenzene.
From the name, there is a benzene ring with 2 bromine groups at 1st and 3rd positions.
The structure will be:
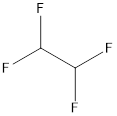
e.
Interpretation:
The structure of 1, 1, 2, 2-tetrafluoroethane needs to be drawn.
Concept introduction:
The organic compounds generally contain carbon atoms. The naming of organic compounds is done by considering three main terms which are prefix, suffix and the root name. The longest chain in the compound is first identified and named that will be the root name for the compound. For example, if there are 3 carbon atoms in the longest chain, the root name will be “prop”. To determine the suffix, functional group should be identified. In the last step, side groups are identified and named. Prefix is used to give number of side groups and substituent’s present on the main carbon chain.
e.
Explanation of Solution
The name of the compound is 1, 1, 2, 2-tetrafluoroethane.
From the name it can be seen that there are 4 fluorine atoms; 2 each at position 1 and 2.
Also, there are 2 carbon atoms in the chain. The structure will be as follows:
Chapter 22 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Genetic Analysis: An Integrated Approach (3rd Edition)
Human Anatomy & Physiology (2nd Edition)
Campbell Essential Biology (7th Edition)
Campbell Biology: Concepts & Connections (9th Edition)
College Physics: A Strategic Approach (3rd Edition)
Cosmic Perspective Fundamentals
- Draw a tetramer of this alternating copolymer.arrow_forwardH I T H HH H -H C. H- Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H CIH H H H HHHH H H 0 ·H H– 冊 CH CHI HH C- H- H H- H H A. H H C H H- -H HH H B. H- -H D. H H H H • H -H E. -H H H HICH T HHH F. H-arrow_forwardPolylactic acid (shown below) is a biodegradable polymer used for food packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forward
- Draw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forwardPoly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forward
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





