
Concept explainers
Table 4 shows data from an experiment to determine the formulas of hydrated barium chloride. Determine the formula for the hydrate and its name.
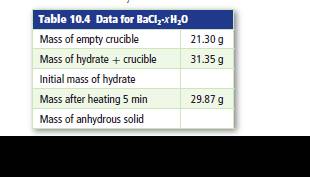
Interpretation:
The formula and name of the given hydrate of barium needs to be determined.
Concept introduction:
Number of water molecules present per molecule of a compound is known as water of crystallization.
Answer to Problem 181A
Name of the hydrate is barium chloride dihydrate.
Formula is BaCl2.2H2O.
Explanation of Solution
Molar mass of BaCl2 is 208 g/mol.
Molar mass of H2O is 18 g/mol.
Total mass of hydrated salt and crucible is 31.35 g.
Mass of the crucible is 21.30 g
Thus,
Mass of hydrated salt
Mass of the salt and crucible after 5 minutes heating is 29.87 g.
Mass of anhydrous salt
Now, number of moles of barium chloride and water can be calculated as follows:
Thus,
Moles of BaCl2
Moles of H2O
Moles ratio of BaCl2:H2O
Formula is BaCl2.2H2O.
Thus, the formula of the hydrate is BaCl2.2H2O which is called barium chloride dihydrate.
Chapter 10 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Brock Biology of Microorganisms (15th Edition)
Biology: Life on Earth (11th Edition)
Campbell Biology (11th Edition)
The Cosmic Perspective (8th Edition)
Introductory Chemistry (6th Edition)
Campbell Biology in Focus (2nd Edition)
- A mixture of 0.568 M H₂O, 0.438 M Cl₂O, and 0.710 M HClO are enclosed in a vessel at 25 °C. H₂O(g) + C₁₂O(g) = 2 HOCl(g) K = 0.0900 at 25°C с Calculate the equilibrium concentrations of each gas at 25 °C. [H₂O]= [C₁₂O]= [HOCI]= M Σ Marrow_forwardWhat units (if any) does the response factor (K) have? Does the response factor (K) depend upon how the concentration is expressed (e.g. molarity, ppm, ppb, etc.)?arrow_forwardProvide the structure, circle or draw, of the monomeric unit found in the biological polymeric materials given below. HO OH amylose OH OH 행 3 HO cellulose OH OH OH Ho HOarrow_forward
- OA. For the structure shown, rank the bond lengths (labeled a, b and c) from shortest to longest. Place your answer in the box. Only the answer in the box will be graded. (2 points) H -CH3 THe b Нarrow_forwardDon't used hand raitingarrow_forwardQuizzes - Gen Organic & Biological Che... ☆ myd21.lcc.edu + O G screenshot on mac - Google Search savings hulu youtube google disney+ HBO zlib Homework Hel...s | bartleby cell bio book Yuzu Reader: Chemistry G periodic table - Google Search b Home | bartleby 0:33:26 remaining CHEM 120 Chapter 5_Quiz 3 Page 1: 1 > 2 > 3 > 6 ¦ 5 > 4 > 7 ¦ 1 1 10 8 ¦ 9 a ¦ -- Quiz Information silicon-27 A doctor gives a patient 0.01 mC i of beta radiation. How many beta particles would the patient receive in I minute? (1 Ci = 3.7 x 10 10 d/s) Question 5 (1 point) Saved Listen 2.22 x 107 222 x 108 3.7 x 108 2.22 x 108 none of the above Question 6 (1 point) Listen The recommended dosage of 1-131 for a test is 4.2 μCi per kg of body mass. How many millicuries should be given to a 55 kg patient? (1 mCi = 1000 μСi)? 230 mCiarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





