
Concept explainers
Interpretation:
The empirical and molecular formula for vitamin D3 should be predicted.
Concept introduction:
The representation of atoms of a compound in simple whole number ratio is known as empirical formula.
Chemical formula or molecular formula represents the type of atoms and number of atoms present in a molecule using numerical subscripts and atomic symbol.
The rules for determining empirical formula is given as follows:
- Determine the mass of elements in given compound.
- Calculate number of moles using molar mass of each compound or element.
- Divide each number of moles by smallest number of mole value calculated in second step.
- Round the value calculated in step 3 to nearest whole number.
Answer to Problem 164A
The empirical and molecular formula for vitamin D3 is
Explanation of Solution
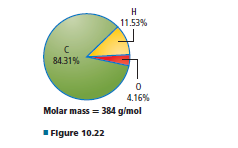
Assuming the percentage is given by mass that means 84.31 g, 4.16 g, and 11.53 g of carbon, oxygen, and hydrogen are present in the vitamin D3.
1. Mass of carbon = 84.31 g
Mass of hydrogen = 11.53 g
Mass of oxygen = 4.16 g
Now, calculating the moles of carbon, hydrogen, and oxygen using their molar mass as follows:
2. Now, dividing each mole value by smallest mole value as follows:
3. Thus, the empirical formula of vitamin D3 is
Now, n that is the ratio of given molar mass and empirical formula mass is calculated as follows:
The empirical formula mass is calculated as follows:
Calculation of n as follows:
Now, the number obtained will be multiplied to empirical formula as follows:
Chapter 10 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
College Physics: A Strategic Approach (3rd Edition)
Microbiology with Diseases by Body System (5th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Introductory Chemistry (6th Edition)
Applications and Investigations in Earth Science (9th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





