
Interpretation:
The conditions required for ionic compounds to conduct electricity and reason for it not being used as conductor always needs to be explained.
Concept introduction:
Molecules are bound together by different types of bonds namely ionic and covalent. Ionic bonds are formed when there is complete transfer of electrons from one atom to another. These atoms either lose or gain electrons to become negatively or positively charged ions. The forces of attraction between these ions causes the ionic bond formation. When molten, the ionic compounds will dissociate to release ions which are free to move around the semi liquid state and hence will be able to conduct electricity.
Answer to Problem 72A
The ionic compound will conduct electricity only in molten form or when dissolved in water.
Explanation of Solution
Ionic bonds involve the transfer of electrons from one atom to the other. The metal atoms loses electrons to form a positively charged ion while the non-metal atoms gains electrons to form negatively charged ions. During the process of donating or gaining electrons, the atoms follow octet rule to attain a stable noble gas configuration.
The ionic compound is a solid but when heated the bonds holding the ions are free to move within the structure. Also, when the solid is dissolved in water, it will break free the lattice points. The ions held at the lattice points are dissociated to free ions. Now, when current is passed through the ions, it will carry the charges. Hence, when electricity is passed through molten or solution containing ionic solids, it is found to conduct electricity.
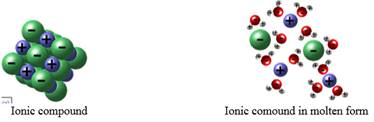
Therefore, ionic compounds will conduct electricity only in molten form or when dissolved in water.
The ionic compound will conduct electricity only in molten form or when dissolved in water.
Chapter 7 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Introductory Chemistry (6th Edition)
College Physics: A Strategic Approach (3rd Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Campbell Biology: Concepts & Connections (9th Edition)
Human Anatomy & Physiology (2nd Edition)
Biology: Life on Earth (11th Edition)
- Please help me with number 1-3. Thank you so much.arrow_forwardDraw the major product of this reaction ingnore the inorganic byproducts. 1. NaOCH2CH3 at 25 C 2. PhCH2Br (1 eq)arrow_forwardAt 90ºC the vapor pressure of ortho-xylene is 20 kPa and that of meta-xylene is 18 kPa. What is the composition of the vapor in equilibrium with a mixture in which the mole fraction of o-xylene is 0.60?arrow_forward
- Draw the products of this reduction of a ketone with sodium borohydride. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, where applicableIgnore any inorganic byproducts. 1) NaBH4 2) HCI/H2O Select to Drawarrow_forwardWhy do you think people who live at high altitudes are advised to add salt to water when boiling food like pasta? What mole fraction of NaCl is needed to raise the boiling point of H2O by 3˚C? Does the amount of salt added to water (typically about one teaspoon to four quarts of water) substantially change the boiling point? (Kb (H2O) = 0.51˚C/molal.)arrow_forwardpls help asaparrow_forward
- pls help asaparrow_forward9. Consider the following galvanic cell: Fe (s) | Fe(NO3)2 (aq) || Sn(NO3)2 (aq) | Sn (s) a. Write an equation for the half reactions occurring at the anode and cathode. b. Calculate the standard cell potential Show all of your work. c. Draw and label the galvanic cell, including the anode and cathode, direction of electron flow, and direction of ion migration.arrow_forwardpls help asaparrow_forward
- 11. Use the equation below to answer the following questions: 2 Al(s) + 3 Cd(NO3)2 (aq) → 2 Al(NO3)3 (aq) + 3 Cd(s) a. What is the net ionic equation for the reaction? b. Which species is a spectator ion in this reaction? Define a spectator ion. c. Identify the oxidizing agent and the reducing agent.arrow_forwardpls help asaparrow_forwardpls help asaparrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





