
Concept explainers
Interpretation:
This is to be shown that iron can form both +2 and +3 ion.
Concept introduction:
The atoms lose or gain electrons to form octet configuration. This process is said to be ionization. Compounds which contain ions are held together by ionic bond that is formed by ions.
Answer to Problem 57A
Iron will lose 2 electrons from 4s orbital to form
Explanation of Solution
Iron belongs to group 8 and has
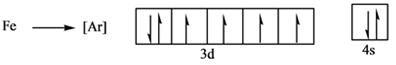
There are 4 shells which are half filled.
As per Madelung’s Law, filling of electrons is in a specific order as the atomic number increases. But as Iron has two outermost or valence electrons in 4s orbital. It can easily lose these two electrons to form
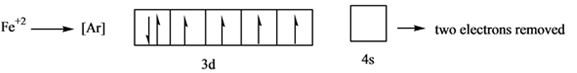
As it is seen that in the next 3d shell which has 6 electrons, there is one orbital having a paired electron while other 4 are half filled. This one of 3d orbital will loose one electron to have all of its 3d orbitals half filled. Hence,
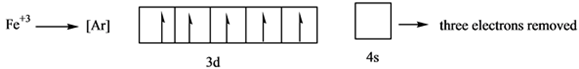
Here it loses 2 electrons from 4s and 1 electron from 3d orbital.
Iron will lose 2 electrons from 4s orbital to form
Chapter 7 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
Microbiology: An Introduction
Chemistry: Structure and Properties (2nd Edition)
Biology: Life on Earth (11th Edition)
Introductory Chemistry (6th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
- For the reaction below, the concentrations at equilibrium are [SO₂] = 0.50 M, [0] = 0.45 M, and [SO3] = 1.7 M. What is the value of the equilibrium constant, K? 2SO2(g) + O2(g) 2SO3(g) Report your answer using two significant figures. Provide your answer below:arrow_forwardI need help with this question. Step by step solution, please!arrow_forwardZn(OH)2(s) Zn(OH)+ Ksp = 3 X 10-16 B₁ = 1 x 104 Zn(OH)2(aq) B₂ = 2 x 1010 Zn(OH)3 ẞ3-8 x 1013 Zn(OH) B4-3 x 1015arrow_forward
- Help me understand this by showing step by step solution.arrow_forwardscratch paper, and the integrated rate table provided in class. our scratch work for this test. Content attribution 3/40 FEEDBACK QUESTION 3 - 4 POINTS Complete the equation that relates the rate of consumption of H+ and the rate of formation of Br2 for the given reaction. 5Br (aq) + BrO3 (aq) + 6H (aq) →3Br2(aq) + 3H2O(l) • Your answers should be whole numbers or fractions without any decimal places. Provide your answer below: Search 尚 5 fn 40 * 00 99+ 2 9 144 a [arrow_forward(a) Write down the structure of EDTA molecule and show the complex structure with Pb2+ . (b) When do you need to perform back titration? (c) Ni2+ can be analyzed by a back titration using standard Zn2+ at pH 5.5 with xylenol orange indicator. A solution containing 25.00 mL of Ni2+ in dilute HCl is treated with 25.00 mL of 0.05283 M Na2EDTA. The solution is neutralized with NaOH, and the pH is adjusted to 5.5 with acetate buffer. The solution turns yellow when a few drops of indicator are added. Titration with 0.02299 M Zn2+ requires 17.61 mL to reach the red end point. What is the molarity of Ni2+ in the unknown?arrow_forward
- A compound has the molecular formula CH40, and shows a strong IR absorption at 2850-3150 cm. The following signals appear in the 'H NMR spectrum: 1.4 ppm (triplet, 6H), 4.0 ppm (quartet, 4H), 6.8 ppm (broad singlet, 4H). Which of the following structures is consistent with these data? Select the single best answer. OCH CH₂ x OCH2CH3 CH₂OCH3 OH CH₂OCH OH CH, OCH₁ CH₂OCH, CH₂OCH HO OH ° CH₂OCH3arrow_forwardpredict the major product while showing me the intermidiate products from each reagent/reagent grouparrow_forwardWhy is it desirable in the method of standard addition to add a small volume of concentrated standard rather than a large volume of dilute standard? An unknown sample of Cu2+ gave an absorbance of 0.262 in an atomic absorption analysis. Then 1.00 mL of solution containing 100.0 ppm (= µg/mL) Cu2+ was mixed with 95.0 mL of unknown, and the mixture was diluted to 100.0 mL in a volumetric flask. The absorbance of the new solution was 0.500. Calculate the concentration of copper ion in the sample.arrow_forward
- What is the relation between the standard deviation and the precision of a procedure? What is the relation between standard deviation and accuracy? The percentage of an additive in gasoline was measured six times with the following results: 0.13, 0.12, 0.16, 0.17, 0.20, 0.11%. Find the 90% and 99% confidence intervals for the percentage of the additive.arrow_forwardIf you measure a quantity four times and the standard deviation is 1.0% of the average, can you be 90% confident that the true value is within 1.2% of the measured average?arrow_forwardWrite down three most common errors in thermogravimetric analysis. Identify them as systematic or random errors and discuss how you can minimize the errors for better results.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





