
Interpretation:
A table showing the atomic and ionic radii of Carbon, Nitrogen, and Oxygen needs to be prepared. Whether the sizes of Carbon, Nitrogen and Oxygen atoms increases or decreases in size when they form bonds in aspartame needs to be explained.
Concept introduction:
Ionic radius can be defined as the radius of the ion in an atom. The atomic radius of a particular element is the measure of the atom’s size. It can also be defined as the distance between its boundary and nucleus of the element
Answer to Problem 72A
Carbon atom will decrease in size on forming bonds in aspartame, whereas nitrogen and oxygen results in increase in the size on forming bonds in aspartame.
Explanation of Solution
The structure of Aspartame is given below:
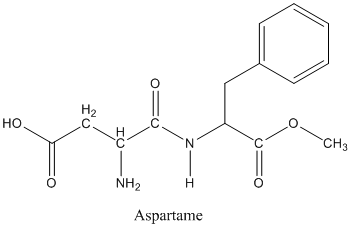
In the bonds, carbon gets partial positive charge. As a result, decrease in radius takes place when ionized. On ionizing or forming bonds, Nitrogen and Oxygen gain electrons and result in the increase in radius.
The table below indicates the atomic and ionic radius of Carbon, Nitrogen and Oxygen atoms.
| Element | Atomic radius | Ionic Radius |
| Carbon | 77 pm | 15 pm |
| Nitrogen | 75 pm | 146 pm |
| Oxygen | 73 pm | 140 pm |
From the data given in the table, one can predict that Carbon atom will decrease in size on forming bonds in aspartame, whereas Nitrogen and Oxygen results in increases in size on forming bonds in aspartame.
Carbon makes bonds after sharing of electrons. It gets partial positive charge. Thus, decrease in radius takes place when ionized. On ionizing or forming bonds, Nitrogen and Oxygen gain electrons and result in the increase in radius.
Therefore, carbon atom will decrease in size on forming bonds in aspartame, whereas Nitrogen and Oxygen results in increases in size on forming bonds in aspartame.
Chapter 6 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Campbell Essential Biology (7th Edition)
Brock Biology of Microorganisms (15th Edition)
Chemistry: The Central Science (14th Edition)
Organic Chemistry (8th Edition)
Campbell Biology (11th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
- Indicate the functions that salt bridges have in batteries.arrow_forwardIn the battery:Pt | H2 (g) | H+ (aq) | Fe2+ (aq) | FeIndicate the cathode and anode.arrow_forwardWrite the equations that occur when the electrode Pb (s) | PbI2 (s) | KI (ac) in a galvanic cell. a) It functions as a positive electrode b) It functions as a negative electrode c) What is the ion with respect to which this electrode is reversible?arrow_forward
- State the formula to find the electromotive force of a battery as a function of the potential of the anode and the cathode.arrow_forwardWhy are normal electrode potentials also called relative electrode potentials?arrow_forwardEasily differentiate between electrochemical potential and Galvani potential.arrow_forward
- Construct a molecular orbital diagram for carbon monoxide. Identify the relevant point group,include all of the appropriate symmetry labels and pictures, and fill in the electrons. Make sure toaccount for the difference in electronegativity between C and O. Hint: CO is substantiallyisoelectronic to N2. (PLEASE DRAW THE ENTIRE MO DIAGRAM!!!)arrow_forwardplease help with hwarrow_forwardhelp me solve this hwarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





