
Interpretation:
A simplified periodic table needs to be sketched labeling the alkali metals, alkaline earth metals,
Concept introduction:
A periodic table consists of groups and periods. The horizontal rows are termed as period and the vertical rows are termed as groups. The elements with similar properties are placed together in a group.
Answer to Problem 33A
The simplified periodic table is shown below and the alkali metals, alkaline earth metals, transition metals, inner transition metals, noble gases and halogens are labeled in the periodic table.
Group 17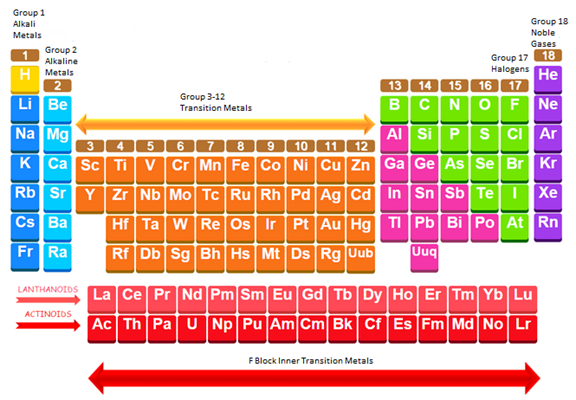
Alkali metals are placed in group 1, alkaline earth metals are placed in group 2, transition metals are placed in between group 3-12, inner transition metals consist of lanthanides and actinides, noble gases are placed in group 17 and halogens are placed in group 18.
Explanation of Solution
The periodic table is divided into alkali metals, alkaline earth metals, transition metals, inner transition metals, noble gases and halogens.
Group 1 and 2 are included in the s-block. Group 1 consists of Alkali metals and Group 2 consists of Alkaline earth metals.
Group 3-12 are included in the d-block. This block consists of transitions metals.
Group 13-18 are included in the p-block. Group 17 consists of halogens and group 18 consists of Nobel gases.
F-block includes the lanthanides and the actinides. These are called the inner transition elements.
The simplified periodic table is shown below:
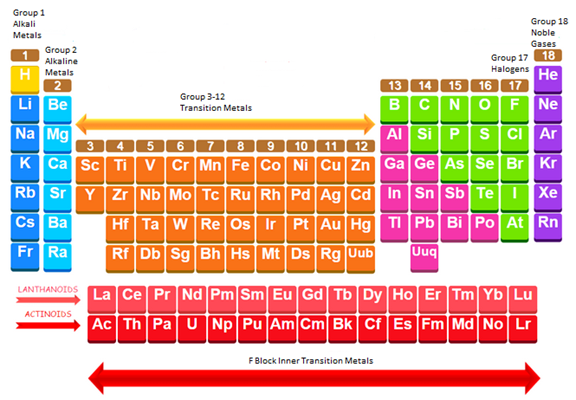
The simplified periodic table is shown below labeling the alkali metals, alkaline earth metals, transition metals, inner transition metals, noble gases and halogens.
Chapter 6 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Microbiology: An Introduction
Genetic Analysis: An Integrated Approach (3rd Edition)
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Applications and Investigations in Earth Science (9th Edition)
Introductory Chemistry (6th Edition)
Anatomy & Physiology (6th Edition)
- Can I please get help with this?arrow_forwardUse the Henderson-Hasselbalch equation to calculate pH of a buffer containing 0.050M benzoic acidand 0.150M sodium benzoate. The Ka of benzoic acid is 6.5 x 10-5arrow_forwardA. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forward
- What is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





