
Concept explainers
(a)
Interpretation:The resonance structure that results from curved arrows mentioned below should be determined.
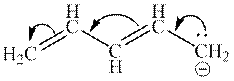
Concept introduction:When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge. Arrow type 1 shows the movement of lone pair toward adjacent atom and then converted into
2. Use only arrow type 3 to move a positive charge for resonance structure of cations. Arrow type 3 is used to move
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
(b)
Interpretation:Whether the use of arrow type 3 in resonance structure of anion skips the important resonance structure or not should be determined.
Concept introduction:When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge. Arrow type 1 shows the movement of lone pair toward adjacent atom and then converted into
2. Use only arrow type 3 to move a positive charge for resonance structure of cations. Arrow type 3 is used to move
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
(c)
Interpretation: The curved arrowsin the resonance structure mentioned below should be added.
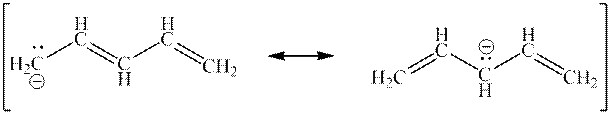
Concept introduction:When one single structure is unable to describe all the properties of single molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for resonance structure of anions in movement of negative charge. Arrow type 1 shows the movement of lone pair toward adjacent atom and then converted into
2. Use only arrow type 3 to move a positive charge for resonance structure of cations. Arrow type 3 is used to move
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be same in all resonance structures.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
- What is the molar mass of a gas that takes three times longer to effuse than helium?arrow_forwardFirst image: I have to show the mecanism (with arows and structures) of the reaction at the bottom. Also I have to show by mecanism why the reaction wouldn't work if the alcohol was primary Second image: I have to show the mecanism (with arrows and structures) for the reaction on the left, where the alcohol A is added fast in one portion its not an examarrow_forwardwhat is the skeletal structure of a tertiary alkyl fluoride with six carbon atoms and no rings.arrow_forward
- One step of glycolysis is a retro-aldol reaction (aldolase) to produce ATP.Below is the aldol reaction of the equilibrium. Show the mechanism for the base catalyzed reaction. *see imagearrow_forwardProvide the missing information. *see imagearrow_forwardWhy does the following reaction lead to poor yields? Correct the reaction. *see imagearrow_forward
- Provide the missing information. *see imagearrow_forwardDraw the mechanism (including all curved arrows for electron movement) showing how the maleicanhydride is attacked by the anthracene and formation of the final Diels Alder product.arrow_forwardProvide the missing information. *see imagearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
