
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5, Problem 23CTQ
Interpretation Introduction
Interpretation: Whether
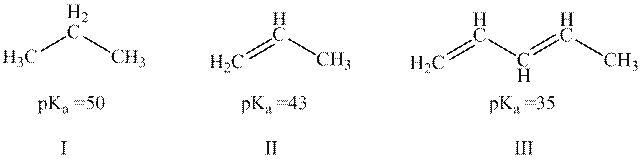
Concept introduction: The dissociation constant of acid represents strength of acid in solution. It is denoted by
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can I get some help with my arrows? I have included what the final outcome needs to look like. #3
Please explain how to calculate the pH.
I'm having trouble with converting lewis diagrams into VSEPR diagrams. I currently have this example of C2BrCl3 which I want to turn into a lewis structure, but I'm not sure what steps I need to do in order to do so. I have the table written down, however, there's two central atoms so what would I do? There seems to be 4 electron domains on the carbon atom and no lone pairs so it would seem like this shape would be tetrahedral. Here's what I have now. Thanks!
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 5 - Which elements on the periodic table (other than...Ch. 5 - You will not find “hydroxide” in the stockroom,...Ch. 5 - Prob. 3CTQCh. 5 - Prob. 4CTQCh. 5 - Prob. 5CTQCh. 5 - Prob. 6CTQCh. 5 - On which do you expect to have a more intense and...Ch. 5 - Prob. 8CTQCh. 5 - Prob. 9CTQCh. 5 - Prob. 10CTQ
Ch. 5 - Prob. 11CTQCh. 5 - Prob. 12CTQCh. 5 - Prob. 13CTQCh. 5 - Prob. 14CTQCh. 5 - Prob. 15CTQCh. 5 - Prob. 16CTQCh. 5 - For each proposed set of resonance structures: a....Ch. 5 - Consider the polarization of the C=O bond in the...Ch. 5 - The C=O double bond is called a “carbonyl bond.”...Ch. 5 - Prob. 20CTQCh. 5 - Prob. 21CTQCh. 5 - Prob. 22CTQCh. 5 - Prob. 23CTQCh. 5 - Prob. 24CTQCh. 5 - Prob. 25CTQCh. 5 - Prob. 26CTQCh. 5 - Prob. 27CTQCh. 5 - Prob. 28CTQCh. 5 - Prob. 29CTQCh. 5 - Prob. 30CTQCh. 5 - Prob. 31CTQCh. 5 - Confirm that there is no legitimate Lewis...Ch. 5 - Draw all resonance structures of the molecule...Ch. 5 - Prob. 34CTQCh. 5 - Prob. 35CTQCh. 5 - Prob. 36CTQCh. 5 - Occasionally, we will see an ionic compound that...Ch. 5 - Prob. 2ECh. 5 - Prob. 3ECh. 5 - Prob. 4ECh. 5 - Is it possible to draw a resonance structure of...Ch. 5 - Prob. 6ECh. 5 - Prob. 7ECh. 5 - Prob. 8ECh. 5 - Phenol (shown below) has a pKa10 . a. Based on pKa...Ch. 5 - Use curved arrows to show the most likely...Ch. 5 - Prob. 12ECh. 5 - Complete each Lewis structure, draw all important...Ch. 5 - Use curved arrows to show the most likely...Ch. 5 - Construct an explanation for why sulfuric acid is...Ch. 5 - Prob. 16ECh. 5 - Prob. 17ECh. 5 - Prob. 18E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- We discussed the solid phase resin using in peptide synthesis. Provide a mechanism, for its formation. DRAW THE MECHANISM.arrow_forwardPlease help. Every time I've asked an expert in the past, it's been wrong :(arrow_forwardPlease help everysingle time ive asked in the past, the solution has been wrongarrow_forward
- Please helparrow_forward(a) 21.8 Name the following compounds. & (b) Br (e) O₂N. (h) H (c) Br (d) NH2 ☑N Br H ہیں Ph (g) OMe бл .0-0.e 21.9 Draw a structural formula for each compound. (a) 2,3-Dinitrotoluene (c) Diphenylmethanol (e) p-Nitroaniline (b) 3-Propylanisole (d) m-Propylphenol (f) Pentabromobenzenearrow_forwardIs this the major product of this reaction?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning