
Concept explainers
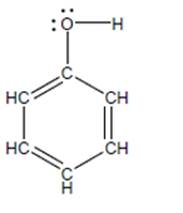
Phenol (shown below) has a
a. Based on
b. Draw the conjugate base of phenol (called phenoxide) including allimportant resonance structures.
c. Construct an explanation for why phenol is a stronger acid than anordinary alcohol. (You may want to consider first why phenoxide is lowerin PE than methoxide
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
- this is an organic chemistry question please answer accordindly!! please post the solution in your hand writing not an AI generated answer please draw the figures and structures if needed to support your explanation hand drawn only!!!! answer the question in a very simple and straight forward manner thanks!!!!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forwardShow work. don't give Ai generated solution and don't copy answer anywherearrow_forwardShow work. don't give Ai generated solutionarrow_forward
- Show work. Don't give Ai generated solutionarrow_forwardA buffered solution containing dissolved aniline, CH,NH2, and aniline hydrochloride, CH, NH, Cl, has a pH of 5.41. Determine the concentration of CH, NH in the solution if the concentration of CH, NH, is 0.305 M. The pK of aniline is 9.13. [CHẠNH] = Calculate the change in pH of the solution, ApH, if 0.375 g NaOH is added to the buffer for a final volume of 1.40 L. Assume that any contribution of NaOH to the volume is negligible. ApH = Marrow_forwardShow work. don't give Ai generated solutionarrow_forward
- Nuclear spin energy levels and electron spin energy levels.arrow_forward1. Polyester Formation a. Draw the structure of the polyester formed (Seabacoyl Chloride + Ethylene Glycol). (Insert scanned hand-drawn structure or ChemDraw image.) b. What molecules are eliminated in this condensation reaction?arrow_forwardDon't used Ai solutionarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
