
Concept explainers
Interpretation: The set of resonance for following anion should be determined.
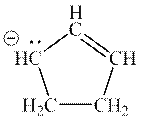
Concept introduction: Lewis structure is a representation of molecules in which dots are shown to represent unshared electrons and lines are shown to represent bonds. These lines and dots represent distribution of electrons in the molecule.
When one single structure is unable to describe all the properties of a single-molecule, a phenomenon called resonance comes into play. This arises when two or more than two Lewis structures are possible for one molecule. All such structures are called resonating structures and have same placement of atoms in them but these have different locations of bond pairs and lone pairs. The resonating structures are inter-convertible with each other. The resultant of all the resonating or contributing structures is called the resonance hybrid.
Rules to form resonance structure are as follows:
1. Use arrow types 1 and 2 for the resonance structure of anions in the movement of negative charge.
2. Use only arrow type 3 to move a positive charge for the resonance structure of cations.
3. The sigma bond should not be broken. Any atom must not move from its place and total number of electrons must be the same in all resonance structures.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
- ASP please....arrow_forwardNonearrow_forwardConsider the structure of 1-bromo-2-fluoroethane. Part 1 of 2 Draw the Newman projection for the anti conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. ✡ ぬ Part 2 of 2 H H F Br H H ☑ Draw the Newman projection for the gauche conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. H F Br H Harrow_forward
- Please help me answer this question. I don't understand how or where the different reagents will attach and it's mostly due to the wedge bond because I haven't seen a problem like this before. Please provide a detailed explanation and a drawing showing how it can happen and what the final product will look like.arrow_forwardWhich of the following compounds is the most acidic in the gas phase? Group of answer choices H2O SiH4 HBr H2Sarrow_forwardWhich of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forward
- Based on the thermodynamics of acetic acid dissociation discussed in Lecture 2-5, what can you conclude about the standard enthalpy change (ΔHo) of acid dissociation for HCl? Group of answer choices You cannot arrive at any of the other three conclusions It is a positive value It is more negative than −0.4 kJ/mol It equals −0.4 kJ/molarrow_forwardPLEASE HELP URGENT!arrow_forwardDraw the skeletal structure corresponding to the following IUPAC name: 7-isopropyl-3-methyldecanearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



