
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5, Problem 24CTQ
Interpretation Introduction
Interpretation: Weaker and stronger base on the basis of
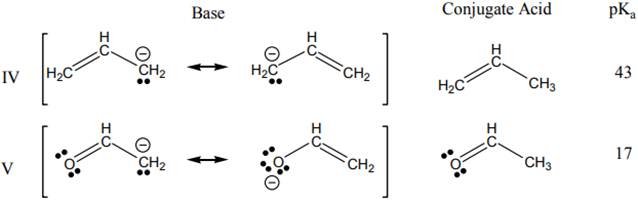
Concept introduction: The dissociation constant of acid represents strength of acid in solution. It is denoted by
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The Concept of Aromaticity
21.15 State the number of 2p orbital electrons in each molecule or ion.
(a)
(b)
(e)
(f)
(c)
(d)
(h)
(i)
DA
(k)
21.16 Which of the molecules and ions given in Problem 21.15 are aromatic according to the
Hückel criteria? Which, if planar, would be antiaromatic?
21.17 Which of the following structures are considered aromatic according to the Hückel
criteria?
---0-0
(a)
(b)
(c)
(d)
(e)
(h)
H
-H
.8.0-
21.18 Which of the molecules and ions from Problem 21.17 have electrons donated by a
heteroatom?
1. Show the steps necessary to make 2-methyl-4-nonene using a
Wittig reaction. Start with triphenylphosphine and an alkyl
halide. After that you may use any other organic or inorganic
reagents.
2. Write in the product of this reaction:
CH3
CH₂
(C6H5)₂CuLi
H₂O+
3. Name this compound properly, including stereochemistry.
H₂C
H3C
CH3
OH
4. Show the step(s) necessary to transform the compound on the
left into the acid on the right.
Bri
CH2
5. Write in the product of this
LiAlH4
Br
H₂C
OH
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 5 - Which elements on the periodic table (other than...Ch. 5 - You will not find “hydroxide” in the stockroom,...Ch. 5 - Prob. 3CTQCh. 5 - Prob. 4CTQCh. 5 - Prob. 5CTQCh. 5 - Prob. 6CTQCh. 5 - On which do you expect to have a more intense and...Ch. 5 - Prob. 8CTQCh. 5 - Prob. 9CTQCh. 5 - Prob. 10CTQ
Ch. 5 - Prob. 11CTQCh. 5 - Prob. 12CTQCh. 5 - Prob. 13CTQCh. 5 - Prob. 14CTQCh. 5 - Prob. 15CTQCh. 5 - Prob. 16CTQCh. 5 - For each proposed set of resonance structures: a....Ch. 5 - Consider the polarization of the C=O bond in the...Ch. 5 - The C=O double bond is called a “carbonyl bond.”...Ch. 5 - Prob. 20CTQCh. 5 - Prob. 21CTQCh. 5 - Prob. 22CTQCh. 5 - Prob. 23CTQCh. 5 - Prob. 24CTQCh. 5 - Prob. 25CTQCh. 5 - Prob. 26CTQCh. 5 - Prob. 27CTQCh. 5 - Prob. 28CTQCh. 5 - Prob. 29CTQCh. 5 - Prob. 30CTQCh. 5 - Prob. 31CTQCh. 5 - Confirm that there is no legitimate Lewis...Ch. 5 - Draw all resonance structures of the molecule...Ch. 5 - Prob. 34CTQCh. 5 - Prob. 35CTQCh. 5 - Prob. 36CTQCh. 5 - Occasionally, we will see an ionic compound that...Ch. 5 - Prob. 2ECh. 5 - Prob. 3ECh. 5 - Prob. 4ECh. 5 - Is it possible to draw a resonance structure of...Ch. 5 - Prob. 6ECh. 5 - Prob. 7ECh. 5 - Prob. 8ECh. 5 - Phenol (shown below) has a pKa10 . a. Based on pKa...Ch. 5 - Use curved arrows to show the most likely...Ch. 5 - Prob. 12ECh. 5 - Complete each Lewis structure, draw all important...Ch. 5 - Use curved arrows to show the most likely...Ch. 5 - Construct an explanation for why sulfuric acid is...Ch. 5 - Prob. 16ECh. 5 - Prob. 17ECh. 5 - Prob. 18E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are the major products of the following reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forwardWhat are the major products of the following enolate alkylation reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forwardA block of zinc has an initial temperature of 94.2 degrees celcius and is immererd in 105 g of water at 21.90 degrees celcius. At thermal equilibrium, the final temperature is 25.20 degrees celcius. What is the mass of the zinc block? Cs(Zn) = 0.390 J/gxdegrees celcius Cs(H2O) = 4.18 J/gx degrees celcusarrow_forward
- Potential Energy (kJ) 1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. AH = -950 kJ AH = 575 kJ (i) Cl₂ (g) + Pt (s) 2C1 (g) + Pt (s) Ea = 1550 kJ (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2240 kJ Ea = 2350 kJ AH = -825 kJ 2600 2400 2200 2000 1800 1600 1400 1200 1000 a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ 800 600 400 200 0 -200- -400 -600- -800- Reaction Progressarrow_forwardCan u help me figure out the reaction mechanisms for these, idk where to even startarrow_forwardHi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward
- Hi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forward
- Draw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forwardDraw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forwardDraw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning