
Interpretation:
All building blocks of matter of the standard model of particle physics needs to be described briefly.
Concept introduction:
All matter around the word is made up of elementary particles, the building block of matter. The main particle occurs in two basic type called quark and leptons.
Answer to Problem 123A
The main group consists of six particles, which are related in pairs or generations. The quarks and leptons are arranged in three generation. The main fundamental forces are of four types but in them the gravity is not explained by The Standard Model.
Explanation of Solution
The group of quark arranged six generations. The six quarks classify as up, down, top, bottom, charm and strange quark. The six leptons are similarly arranged in three generation. The six groups are as electrons, electron- neutrino, the muon, muon-neutrino, tau and tau-neutrino. The nature of muon, electron and tau are electrical charge while the neutrino is neutral in nature.
Now the other four fundamental forces are named as bonson. They classify as strong forces, weak forces,
Where the strong forces are carried by gluon, and the electromagnetic forces are carried by the photon, W, Z.
But the gravitational forces infinite range that is not explained by The Standard Model of physics.
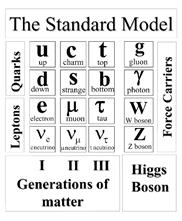
These particles are the known as building basic unit of the matter. There are different types of forces which participated in maintaining the attraction between them. All these particles are experimentally detected by The Standard Model.
Chapter 4 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Microbiology: An Introduction
Applications and Investigations in Earth Science (9th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Human Physiology: An Integrated Approach (8th Edition)
Anatomy & Physiology (6th Edition)
College Physics: A Strategic Approach (3rd Edition)
- Calculating the pH at equivalence of a titration Try Again Your answer is incorrect. 0/5 a A chemist titrates 70.0 mL of a 0.7089 M hydrocyanic acid (HCN) solution with 0.4574M KOH solution at 25 °C. Calculate the pH at equivalence. The pK of hydrocyanic acid is 9.21. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = 11.43] G 00. 18 Ar B•arrow_forwardBiological Macromolecules Naming and drawing the products of aldose oxidation and reduction aw a Fischer projection of the molecule that would produce L-ribonic acid if it were subjected to mildly oxidizing reaction conditions. Click and drag to start drawing a structure. X AP ‡ 1/5 Naor Explanation Check McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibilarrow_forward● Biological Macromolecules Identifying the parts of a disaccharide Take a look at this molecule, and then answer the questions in the table below it. CH2OH O H H H OH OH OH H H CH2OH H O OH H OH H H H H OH Is this a reducing sugar? Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. Explanation Check O yes X O no ○ yes O no Uarrow_forward
- The aim of the lab is to measure the sodium content from tomato sauce using the Mohr titration method. There are two groups being: Regular Tomato sauce & Salt Reduced tomato sauce QUESTION: State how you would prepare both Regular & Salt reduced tomato sauce samples for chemical analysis using the Mohr titration methodarrow_forwardUsing the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds faster at temperatures above -48. °C. ΔΗ is (pick one) ✓ AS is (pick one) B This reaction is spontaneous except below 114. °C but proceeds at a slower rate below 135. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is C This reaction is exothermic and proceeds faster at temperatures above -43. °C. (pick one) AS is (pick one) v Х 5 ? 18 Ararrow_forwardion. A student proposes the following Lewis structure for the perchlorate (CIO) io : :0: : Cl : - - : :0: ك Assign a formal charge to each atom in the student's Lewis structure. atom central O formal charge ☐ top O ☐ right O ☐ bottom O ☐ Cl ☐arrow_forward
- Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure Yes. Is the proposed Lewis structure reasonable? Cl- : 2: :Z: :Z: N—N : 0: C C1: O CO No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". ☑arrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions ΔΗ is (pick one) A This reaction is faster above 103. °C than below. AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous only above -9. °C. AS is (pick one) ΔΗ is (pick one) C The reverse of this reaction is always spontaneous. AS is (pick one) 18 Ararrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds slower at temperatures below 41. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 94. °C. AS is (pick one) This reaction is always spontaneous, but ΔΗ is (pick one) C proceeds slower at temperatures below −14. °C. AS is (pick one) Х 00. 18 Ar 무ㅎ B 1 1arrow_forward
- Draw the product of the reaction shown below. Ignore inorganic byproducts. + H CH3CH2OH HCI Drawingarrow_forwardplease explain this in simple termsarrow_forwardK Most Reactive Na (3 pts) Can the metal activity series (shown on the right) or a standard reduction potential table explain why potassium metal can be prepared from the reaction of molten KCI and Na metal but sodium metal is not prepared from the reaction of molten NaCl and K metal? Show how (not). Ca Mg Al с Zn Fe Sn Pb H Cu Ag Au Least Reactivearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





