
Interpretation:
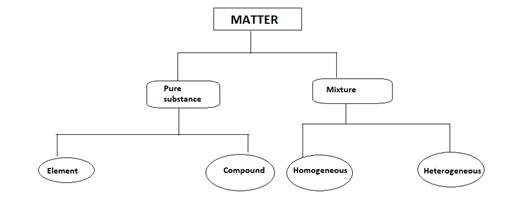
The map showing the relationship between matter, elements, pure substance, compounds and homogeneous and heterogeneous mixtures needs to be explained.
Concept introduction:
The
Explanation of Solution
Substances that cannot be separated into other constituents and have a particular composition is known as pure substances.
Pure substances are further classified into two types:
- Mixture
- Compounds
Pure substances are pure in nature. They cannot be separated by physical process.
Examples of pure substances are gold and pure water.
- Elements are made up only one type of atoms.
For example, Cu, Na, Ne, etc.
- Compounds are made up of more than on the type of atoms.
For example, HCl, PCl5, etc.
- Mixtures are a combination of two or more pure substances.
Mixtures are impure in nature. They can be separated by several methods such as magnetic separation, evaporation, etc.
Examples of mixtures are oil and water, sand and sugar
Mixtures are of two types:
- Homogeneous mixture
- Heterogeneous mixture.
The mixtures in which the components are mixed uniformly are known as homogeneous mixtures. It is also known as solution. The homogeneous mixture cannot be judge by seeing it. They have only one phase. The homogeneous mixtures cannot be separated physically.
Examples of a homogeneous mixture are rainwater, vinegar, etc.
The mixtures in which the components are not mixed uniformly are known as heterogeneous mixtures. The heterogeneous mixture can be judge by seeing it. They have two or more phases or layers. The heterogeneous mixtures can be separated physically.
Examples of a heterogeneous mixture is a mixture of sand and sodium chloride.
The map is shown below:
Chapter 3 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Microbiology with Diseases by Body System (5th Edition)
Campbell Biology (11th Edition)
The Cosmic Perspective (8th Edition)
Organic Chemistry (8th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
College Physics: A Strategic Approach (3rd Edition)
- b) Use curved arrows to show the reaction of the radical with hydrogen bromide. Br: Br H .. Answer Bankarrow_forwardIndicate the reaction products when CH3COCH2COOCH2COOC2H5 (ethyl acetoacetoacetate) reacts with 1º OH-/H2O and 2º H3O+arrow_forwardDraw the formula of the compound 4-cyclohexyl butanamide?arrow_forward
- What is the formula of the compound 3-isopropylcyclopentane-1-carbonyl chloride?arrow_forwardIndicate the products of the reaction between CH3COCH2COONa (Sodium acetoacetate) and BrCH2COOC2H5arrow_forwardIndicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forward
- Indicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forwardPrimary, Secondary, and Tertiary Alcohols O-H O-H O-H R₁-C-H R₁-C-H R₁-C-R₁ H R₂ R₂ Primary Alcohol Secondary Alcohol ChemistryLearner.com R stands for Carbon group like ethyl methyl propyl Tertiary Alcohol If 1 carbon group with two H attached to alcoholic carbon, then primary If 2 carbon group and 1 H are attached to alcoholic carbon, then secondary IF 3 carbon group and no H attach to alcoholic carbon then tertiary. The bottom line Starting "Weak" oxidant material PCC, DMP, Swern, etc Primary alcohol Aldehyde OH Secondary alcohol Ketone OH "Strong" oxidant KMnO4, H₂CrO4 (or equivalent) OH Carboxylic acid 요 Ketone No reaction No reaction Tertiary alcohol 1. Is ethanol a primary, secondary, or tertiary alcohol? Write out the structures of ethanol and any oxidation products of ethanol. If there is more than one oxidation product, give the structure of each of the products. 2. Is 2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-propanol and any…arrow_forwardFormulate the reaction: Naphthalene with CrO3 in acetic acid at 25ºCarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





