
Interpretation:
The full structure of the DNA dinucleotide C – T has to be drawn and 5’ and 3’ ends has to be identified.
Concept Introduction:
Composition of
Sugar: In both DNA and RNA, sugar portion is found. In DNA, the sugar is D-ribose, where at 2’hydroxyl group is absent and in RNA, the hydroxyl group is present at 2’.
Nitrogenous bases: Five types of nitrogenous bases (has unique one-letter code A, G, T, U, and C) are derived from two parent compounds called purine and pyrimidine. The purine derivatives are Adenine and Guanine are two fused nitrogen containing rings. The pyrimidine derivatives are Thymine, Cytosine, and Uracil are only one nitrogen containing six-membered ring. Adenine, Guanine, Thymine, and Cytosine are the nitrogenous bases present in DNA. Adenine, Guanine, Cytosine and Uracil are the nitrogenous bases present in RNA.
Nucleotide: (Nucleoside + phosphate)
Nucleotides are the building blocks of nuclei acids; monomers of DNA and RNA
Numbering the atoms in sugar and base rings:
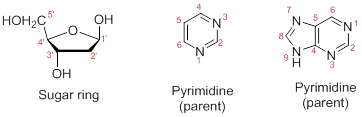
In order to distinguish the atoms in the sugar of a nucleoside and atoms of a base ring, numbers without prime is used for atoms in the base ring and numbers with prime used for the atoms in the sugar ring.
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
- 4. (15 points) Consider a spherical organism of radius ro within which respiration occurs at a uniform volumetric rate of That is, oxygen (species A) consumption is governed by a first- order reaction, homogeneous chemical reaction. a. If a molar concentration of CA(ro) = CA,o is maintained at the surface of the organism, obtain an expression for the radial distribution of oxygen, CA(r), within the organism. Hint: To simplify solution of the species diffusion equation, invoke the transformation y = rCA. b. Obtain an expression for the rate of oxygen consumption within the organism. c. Consider an organism of radius ro = 0.10 mm and a diffusion coefficient of DAB = 108 m2/s. If CA, o = 5 x105 kmol/m3 and k1 20 s1, estimate the corresponding value of the molar concentration at the center of the organism. What is the rate of oxygen consumption by the organism?arrow_forward3. (15 points) Living cells homogeneously distributed (immobilized) with an agarose gel require glucose to survive. An important aspect of the biochemical system design is the effective diffusion coefficient of glucose (A) into the cell- immobilized gel. Consider the experiment shows below where a slab of the cell-immobilized gel of 1.0cm thickness is placed within a well-mixed aqueous solution of glucose maintained at a concentration of 50 mmol/L. The glucose consumption within the cell-immobilized gel proceeds by a zero-order process given by R₁ = -0.05 mmol/(L min). The solubilities of glucose in both the water and the gel are the same; that is, the concentration of the glucose on the water side of the water-gel interface is equal to the concentration of the glucose on the gel side of the water gel interface. A syringe is mounted at the center of the gel carefully excises a tiny sample of the gel for glucose analysis. A Well mixed solution Constant concentration 50nmol/L Living…arrow_forwardTwo tetrapeptides were isolated from a possum's sweat glands. These peptides were sequenced using Edman degradation and the following 2 sequences were obtained: Gly-Asp-Ala-Leu Gly-Asp-Asp-Leu Can you please help show the titration curve for both of these peptides and calculate the PI?arrow_forward
- Two tetrapeptides were isolated from a possum's sweat glands. These peptides were sequenced using Edman degradation and the following 2 sequences were obtained: Gly-Asp-Ala-Leu Gly-Asp-Asp-Leu What is the structure of the PTH derivative produced during the last round of amino acid sequencing?arrow_forwardWhat is the primary sequence of this undecapeptide? Also, if x-ray crystallography shows a highly stable hairpin turn within the polypeptide, what about the primary sequence explains this structural feature?arrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. H H ⚫OH HO- -H H- -OH H- -OH CH2OH Ag*, NH4OH, H2O Draw Fischer Projectionarrow_forward
- Draw the product of this reaction. Ignore inorganic byproducts. H₂O -OH H ⚫OH HO H HO- CH2OH Cu2+ Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. H、 H -OH H ⚫OH H -OH CH2OH Fehlings' solution ⑤ Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. HO C=0 H ⚫OH H ⚫OH HO- H HO H CH2OH Tollens' solution Draw Fischer Projectionarrow_forward
- Draw the product of this reaction. Ignore inorganic byproducts. H-C=O HO H HO H H- ⚫OH HO H CH2OH HNO3, H2O Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. HO HO- HO H HO ∙H HO CH2OH NaBH4, CH3OH Draw Fischer Projectionarrow_forwardDraw the product of this reaction. Ignore inorganic byproducts. Но сво HO H HO H H OH H -OH CH2OH H2 Pd Draw Fischer Projectionarrow_forward
 Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
 Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781337408332Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781337408332Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning





