
Concept explainers
(a)
Interpretation: A cyclic compound that is an isomer of trans-2-butene needs to be drawn.
Concept Introduction : Structural isomers have the same molecular formula. The bonding pattern and the arrangement of the atoms are different in different isomers.
(a)
Answer to Problem 49E

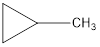
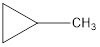
Explanation of Solution
The formula of trans-2-butane is C4H8. The cyclic compounds that is an isomer of trans-2-butene are as follows:

(b)
Interpretation: An ester that is an isomer of propanoic acid needs to be drawn.
Concept Introduction : Structural isomers have the same molecular formula. The bonding pattern and the arrangement of the atoms are different in different isomers.
(b)
Answer to Problem 49E
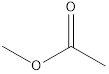
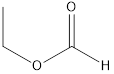
Methyl ethanoate ethyl methanoate
Explanation of Solution
The molecular formula of propanoic acid is
Esters which are isomers of propanoic acid are as follows:
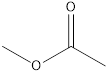
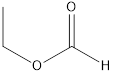
Methyl ethanoate ethyl methanoate
(c)
Interpretation: A
Concept Introduction : Structural isomers have the same molecular formula. The bonding pattern and the arrangement of the atoms are different in different isomers.
(c)
Answer to Problem 49E
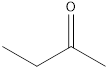
2-butanone
Explanation of Solution
The molecular formula of butanal is
A ketone that is an isomer of butanal is as follows:
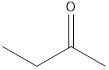
2-butanone
(d)
Interpretation: Secondary
Concept Introduction : Structural isomers have the same molecular formula. The bonding pattern and the arrangement of the atoms are different in different isomers.
(d)
Answer to Problem 49E
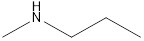

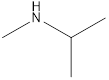
Methyl-propyl-amine Diethyl-amine Isopropyl-methyl-amine
Explanation of Solution
Secondary amine that is an isomer of butylamine are,
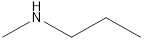

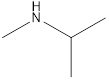
Methyl-propyl-amine Diethyl-amine Isopropyl-methyl-amine
(e)
Interpretation: A tertiary amine that is an isomer of butylamine needs to be drawn.
Concept Introduction : Structural isomers have the same molecular formula. The bonding pattern and the arrangement of the atoms are different in different isomers.
(e)
Answer to Problem 49E
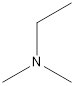
Ethyl-dimethyl-amine
Explanation of Solution
A tertiary amine that is an isomer of butylamine is as follows:
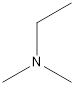
Ethyl-dimethyl-amine
(f)
Interpretation: An ether that is an isomer of 2-methyl-2-propanol needs to be drawn.
Concept Introduction : Structural isomers have the same molecular formula. The bonding pattern and the arrangement of the atoms are different in different isomers.
(f)
Answer to Problem 49E
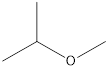


2-Methyoxy-propane Ethoxy-ethane 1-Methoxy-propane
Explanation of Solution
The formula of 2-methyl-2-propanol is
Ethers that are isomers of 2-methyl-2-propanol are as follows:
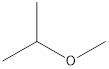


2-Methyoxy-propane Ethoxy-ethane 1-Methoxy-propane
(g)
Interpretation: A secondary alcohol that is an isomer of 2-methyl-2-propanol needs to be drawn.
Concept Introduction : Structural isomers have the same molecular formula. The bonding pattern and the arrangement of the atoms are different in different isomers.
(g)
Answer to Problem 49E
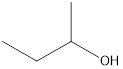
2-butanol
Explanation of Solution
The formula of 2-methyl-2-propanol is
Secondary alcohol that is an isomer of 2-methyl-2-propanol is as follows:
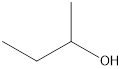
2-butanol
Want to see more full solutions like this?
Chapter 21 Solutions
Chemical Principles
- Please help me solve this reaction.arrow_forwardIndicate the products obtained by mixing 2,2-dimethylpropanal with acetaldehyde and sodium ethoxide in ethanol.arrow_forwardSynthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- If possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIndicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forward
- We mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forwardIndicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


