
Concept explainers
(a)
Interpretation: The test to distinguish
Concept Introduction :
(a)
Answer to Problem 66E
Explanation of Solution
When bromine adds to alkene, the red colour of bromine lost due to boning of bromine to alkene. This is an indication of alkene.
The reaction is as follows:
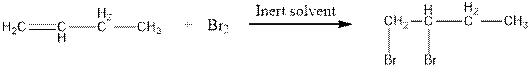
But on addition of bromine to alkane, its red colour will not no away.
(b)
Interpretation: The test to distinguish  needs to be explained.
needs to be explained.
Concept Introduction :
(b)
Answer to Problem 66E
 by reacting with neutral
by reacting with neutral
Explanation of Solution
On reaction of butanoic acid with neutral
But ketone does not show this test.
(c)
Interpretation: The test that distinguishes  needs to be explained.
needs to be explained.
Concept Introduction : In iodoform reaction, the organic compound is added to mixture of iodine and dilutes NaOH.
(c)
Answer to Problem 66E
Iodoform test distinguishes between 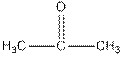
Explanation of Solution
Iodoform test distinguishes between alcohol and ketone.
Ketones form yellow precipitate of iodoform in iodoform test.
The reaction is shown below.
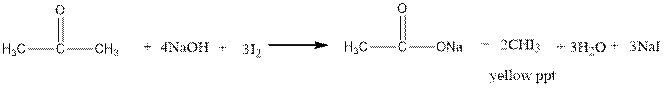
The alcohols do not undergo iodoform test.
(d)
Interpretation: The test that distinguishes
Concept Introduction: In carbylamines reaction,
(d)
Answer to Problem 66E
Carbylamine test distinguishes
Explanation of Solution
Amines react with chloroform and a solution of potassium hydroxide (KOH) to produce a bad odour (Carbyamine test) but ether does not undergo this test.
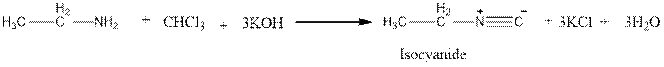
But ether does not undergo this reaction.
Want to see more full solutions like this?
Chapter 21 Solutions
Chemical Principles
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forwardCan I please get help with this?arrow_forwardCan I please get help with this?arrow_forward
- Use the Henderson-Hasselbalch equation to calculate pH of a buffer containing 0.050M benzoic acidand 0.150M sodium benzoate. The Ka of benzoic acid is 6.5 x 10-5arrow_forwardA. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forward
- Can I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





