
Concept explainers
(a)
Interpretation: The
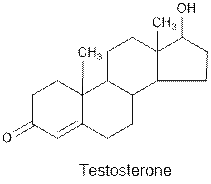
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
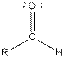 | |
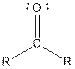 | |
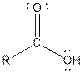 | |
| Ester | 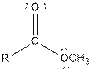 |
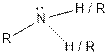 | |
| Alcohol | 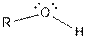 |
(a)
Answer to Problem 38E
Testosterone contains ketone and alcohol functional group.
Explanation of Solution
The general structure for ketone and alcohol are as follows:
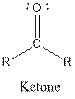
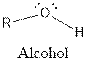
Hence, ketone and alcohol functional groups are present in the given compound as:
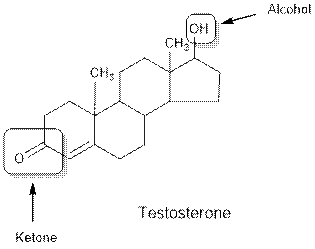
(b)
Interpretation: The functional groups present in the given compound needs to be determined.
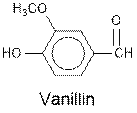
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 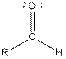 |
| Ketone | 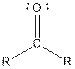 |
| Carboxylic acid | 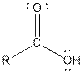 |
| Ester | 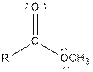 |
| Amine | 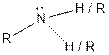 |
| Ether | 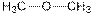 |
(b)
Answer to Problem 38E
Vanillin contains alcohol, ether and aldehyde functional groups.
Explanation of Solution
The general structure for ether, aldehyde and alcohol are as follows:
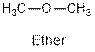
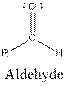
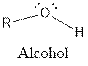
Hence, ether, aldehyde and alcohol functional groups are present in the given compound as:
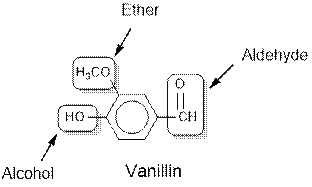
(c)
Interpretation: The functional groups present in the given compound needs to be determined.
Concept Introduction : Organic molecules have some structural features in addition to
Table given below gives information about some common functional groups:
| Type of Compound | General Structure |
| Aldehyde | 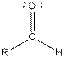 |
| Ketone | 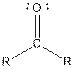 |
| Carboxylic acid | 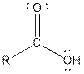 |
| Ester | 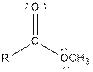 |
| Amine | 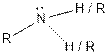 |
| Amide | 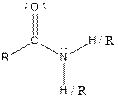 |
(c)
Answer to Problem 38E
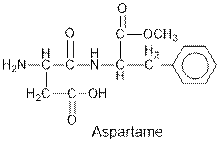
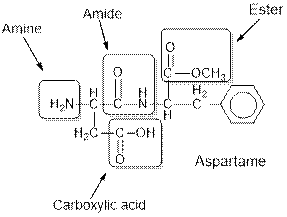
Aspartame contains carboxylic acid, amine, amide and ester functional groups.
Explanation of Solution
The general structure for ether, aldehyde and alcohol are as follows:
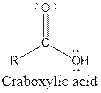

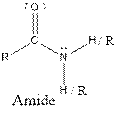
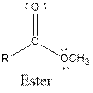
Hence, carboxylic acid, amine, amide and ester functional groups are present in the given compound as:
Want to see more full solutions like this?
Chapter 21 Solutions
Chemical Principles
- 4. For the following complexes, draw the structures and give a d-electron count of the metal: a) Tris(acetylacetonato)iron(III) b) Hexabromoplatinate(2-) c) Potassium diamminetetrabromocobaltate(III) (6 points)arrow_forward2. Calculate the overall formation constant for [Fe(CN)6]³, given that the overall formation constant for [Fe(CN)6] 4 is ~1032, and that: Fe3+ (aq) + e = Fe²+ (aq) E° = +0.77 V [Fe(CN)6]³ (aq) + e¯ = [Fe(CN)6] (aq) E° = +0.36 V (4 points)arrow_forward5. Consider the compounds shown below as ligands in coordination chemistry and identify their denticity; comment on their ability to form chelate complexes. (6 points) N N A B N N N IN N Carrow_forward
- 1. Use standard reduction potentials to rationalize quantitatively why: (6 points) (a) Al liberates H2 from dilute HCl, but Ag does not; (b) Cl2 liberates Br2 from aqueous KBr solution, but does not liberate C12 from aqueous KCl solution; c) a method of growing Ag crystals is to immerse a zinc foil in an aqueous solution of AgNO3.arrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1 1. PPh3 2. n-BuLi 3 2 • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure. Xarrow_forwardWhat is the missing reactant R in this organic reaction? N N H3O+ +R + • Draw the structure of R in the drawing area below. • Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. Click and drag to start drawing a structure. fmarrow_forward
- The product on the right-hand side of this reaction can be prepared from two organic reactants, under the conditions shown above and below the arrow. Draw 1 and 2 below, in any arrangement you like. 1+2 NaBH3CN H+ N Click and drag to start drawing a structure. 5arrow_forwardAssign this HSQC Spectrum ( please editing clearly on the image)arrow_forward(a 4 shows scanning electron microscope (SEM) images of extruded actions of packing bed for two capillary columns of different diameters, al 750 (bottom image) and b) 30-μm-i.d. Both columns are packed with the same stationary phase, spherical particles with 1-um diameter. A) When the columns were prepared, the figure shows that the column with the larger diameter has more packing irregularities. Explain this observation. B) Predict what affect this should have on band broadening and discuss your prediction using the van Deemter terms. C) Does this figure support your explanations in application question 33? Explain why or why not and make any changes in your answers in light of this figure. Figure 4 SEM images of sections of packed columns for a) 750 and b) 30-um-i.d. capillary columns.³arrow_forward
- fcrip = ↓ bandwidth Il temp 32. What impact (increase, decrease, or no change) does each of the following conditions have on the individual components of the van Deemter equation and consequently, band broadening? Increase temperature Longer column Using a gas mobile phase instead of liquid Smaller particle stationary phase Multiple Paths Diffusion Mass Transferarrow_forward34. Figure 3 shows Van Deemter plots for a solute molecule using different column inner diameters (i.d.). A) Predict whether decreasing the column inner diameters increase or decrease bandwidth. B) Predict which van Deemter equation coefficient (A, B, or C) has the greatest effect on increasing or decreasing bandwidth as a function of i.d. and justify your answer. Figure 3 Van Deemter plots for hydroquinone using different column inner diameters (i.d. in μm). The data was obtained from liquid chromatography experiments using fused-silica capillary columns packed with 1.0-μm particles. 35 20 H(um) 큰 20 15 90 0+ 1500 100 75 550 01 02 594 05 μ(cm/sec) 30 15 10arrow_forwardelow are experimentally determined van Deemter plots of column efficiency, H, vs. flow rate. H is a quantitative measurement of band broadening. The left plot is for a liquid chromatography application and the night is for gas chromatography. Compare and contrast these two plots in terms of the three band broadening mechanisms presented in this activity. How are they similar? How do they differ? Justify your answers.? 0.4 H (mm) 0.2 0.1- 0.3- 0 0.5 H (mm) 8.0 7.0 6.0 5.0 4.0- 3.0 T +++ 1.0 1.5 0 2.0 4.0 Flow Rate, u (cm/s) 6.0 8.0 Flow Rate, u (cm/s)arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





