
Concept explainers
(a)
Interpretation:
Valence bond description of the bonding in the given compound has to be given along with its orbital diagram for the free metal ion and metal ion in the complex. The hybrid orbitals which are used by the metal ion for bonding should be indicated and the number of unpaired electron should be specified.
Concept introduction:
Coordination compounds are a special class of compounds in which the metal atoms or ions are bounded to a number of anions or neutral molecules.
A coordination compound can be simply represented as follows,
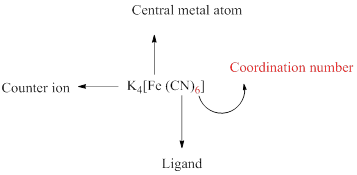
Coordination number of a complex is defined as the number of ligand donor atoms to which the metal is directly bonded.
According to
These hybridized orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding.
Inner orbital complex are coordination compounds having a central metal atom that undergoes hybridization of atomic orbital including the inner d (
Outer orbital complex are coordination compounds having a central metal atom that undergoes hybridization of atomic orbital including the outer most d (
(b)
Interpretation:
Valence bond description of the bonding in the given compound has to be given along with its orbital diagram for the free metal ion and metal ion in the complex. The hybrid orbitals which are used by the metal ion for bonding should be indicated and the number of unpaired electron should be specified.
Concept introduction:
Coordination compounds are a special class of compounds in which the metal atoms or ions are bounded to a number of anions or neutral molecules.
A coordination compound can be simply represented as follows,

Coordination number of a complex is defined as the number of ligand donor atoms to which the metal is directly bonded.
According to valence bond theory, the metal atom or ion under the influence of ligands can use its
These hybridized orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding.
Inner orbital complex are coordination compounds having a central metal atom that undergoes hybridization of atomic orbital including the inner d (
Outer orbital complex are coordination compounds having a central metal atom that undergoes hybridization of atomic orbital including the outer most d (
(c)
Interpretation:
Valence bond description of the bonding in the given compound has to be given along with its orbital diagram for the free metal ion and metal ion in the complex. The hybrid orbitals which are used by the metal ion for bonding should be indicated and the number of unpaired electron should be specified.
Concept introduction:
Coordination compounds are a special class of compounds in which the metal atoms or ions are bounded to a number of anions or neutral molecules.
A coordination compound can be simply represented as follows,

Coordination number of a complex is defined as the number of ligand donor atoms to which the metal is directly bonded.
According to valence bond theory, the metal atom or ion under the influence of ligands can use its
These hybridized orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding.
Inner orbital complex are coordination compounds having a central metal atom that undergoes hybridization of atomic orbital including the inner d (
Outer orbital complex are coordination compounds having a central metal atom that undergoes hybridization of atomic orbital including the outer most d (
(d)
Interpretation:
Valence bond description of the bonding in the given compound has to be given along with its orbital diagram for the free metal ion and metal ion in the complex. The hybrid orbitals which are used by the metal ion for bonding should be indicated and the number of unpaired electron should be specified.
Concept introduction:
Coordination compounds are a special class of compounds in which the metal atoms or ions are bounded to a number of anions or neutral molecules.
A coordination compound can be simply represented as follows,

Coordination number of a complex is defined as the number of ligand donor atoms to which the metal is directly bonded.
According to valence bond theory, the metal atom or ion under the influence of ligands can use its
These hybridized orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding.
Inner orbital complex are coordination compounds having a central metal atom that undergoes hybridization of atomic orbital including the inner d (
Outer orbital complex are coordination compounds having a central metal atom that undergoes hybridization of atomic orbital including the outer most d (
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
General Chemistry: Atoms First
- 11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forwardUnder aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardAssign these COSY Spectrumarrow_forward
- Assign these C-NMR and H-NMR Spectrumarrow_forwardPredict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forwardPredict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forward
- Predict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forward
- Predict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





