
General Chemistry: Atoms First
2nd Edition
ISBN: 9780321809261
Author: John E. McMurry, Robert C. Fay
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 20.26CP
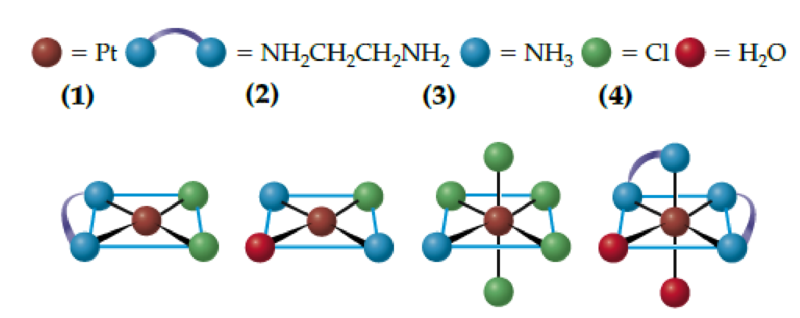
What is the systematic name for each of the following molecules or ions? Include cis or trans prefixes for diastereoisomers. Platinum is Pt(II) in square planar complexes and Pt(IV) in octahedral complexes.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
State two variables on which the transport number in electrochemistry depends.
Among the methods for measuring transport numbers are the Hittorf method and the moving surface method. Let's explain them briefly.
can you help me solve and highlight these hw
Chapter 20 Solutions
General Chemistry: Atoms First
Ch. 20.1 - Write the electron configuration of the metal in...Ch. 20.1 - Prob. 20.2CPCh. 20.3 - Prob. 20.3CPCh. 20.4 - Prob. 20.4PCh. 20.4 - Prob. 20.5PCh. 20.5 - Prob. 20.6PCh. 20.5 - Prob. 20.7PCh. 20.6 - Prob. 20.8PCh. 20.7 - Name each of the following: (a) [Cu(NH3)4]SO4, a...Ch. 20.7 - Prob. 20.10P
Ch. 20.8 - Prob. 20.11PCh. 20.8 - Prob. 20.12CPCh. 20.9 - Prob. 20.13PCh. 20.9 - Prob. 20.14CPCh. 20.9 - Prob. 20.15PCh. 20.10 - Prob. 20.16PCh. 20.11 - Prob. 20.17PCh. 20.12 - Prob. 20.18PCh. 20.12 - Prob. 20.19PCh. 20.12 - Prob. 20.20PCh. 20.12 - Prob. 20.21PCh. 20 - Prob. 20.22CPCh. 20 - Prob. 20.23CPCh. 20 - Prob. 20.24CPCh. 20 - Prob. 20.25CPCh. 20 - What is the systematic name for each of the...Ch. 20 - Prob. 20.27CPCh. 20 - Prob. 20.28CPCh. 20 - Prob. 20.29CPCh. 20 - Predict the crystal field energy-level diagram for...Ch. 20 - Prob. 20.31CPCh. 20 - Use the periodic table to give the electron...Ch. 20 - Prob. 20.33SPCh. 20 - Prob. 20.34SPCh. 20 - Prob. 20.35SPCh. 20 - Prob. 20.36SPCh. 20 - Prob. 20.37SPCh. 20 - Prob. 20.38SPCh. 20 - Prob. 20.39SPCh. 20 - What is the lanthanide contraction, and why does...Ch. 20 - The atomic radii of zirconium and hafnium are...Ch. 20 - Calculate the sum of the first two ionization...Ch. 20 - Prob. 20.43SPCh. 20 - Prob. 20.44SPCh. 20 - Prob. 20.45SPCh. 20 - Prob. 20.46SPCh. 20 - Prob. 20.47SPCh. 20 - Prob. 20.48SPCh. 20 - Prob. 20.49SPCh. 20 - Prob. 20.50SPCh. 20 - Prob. 20.51SPCh. 20 - Prob. 20.52SPCh. 20 - Prob. 20.53SPCh. 20 - Prob. 20.54SPCh. 20 - Prob. 20.55SPCh. 20 - Write a balanced equation for the industrial...Ch. 20 - Prob. 20.57SPCh. 20 - Prob. 20.58SPCh. 20 - Prob. 20.59SPCh. 20 - Prob. 20.60SPCh. 20 - Prob. 20.61SPCh. 20 - Prob. 20.62SPCh. 20 - Prob. 20.63SPCh. 20 - Prob. 20.64SPCh. 20 - Prob. 20.65SPCh. 20 - Prob. 20.66SPCh. 20 - Prob. 20.67SPCh. 20 - Prob. 20.68SPCh. 20 - Prob. 20.69SPCh. 20 - Prob. 20.70SPCh. 20 - Prob. 20.71SPCh. 20 - Prob. 20.72SPCh. 20 - Prob. 20.73SPCh. 20 - What is the formula of a complex that has each of...Ch. 20 - What is the formula, including the charge, for...Ch. 20 - Prob. 20.76SPCh. 20 - Prob. 20.77SPCh. 20 - Prob. 20.78SPCh. 20 - Prob. 20.79SPCh. 20 - Prob. 20.80SPCh. 20 - Prob. 20.81SPCh. 20 - What is the systematic name for each of the...Ch. 20 - Prob. 20.83SPCh. 20 - Prob. 20.84SPCh. 20 - Prob. 20.85SPCh. 20 - Prob. 20.86SPCh. 20 - Prob. 20.87SPCh. 20 - Prob. 20.88SPCh. 20 - Tell how many diastereoisomers are possible for...Ch. 20 - Which of the following complexes are chiral? (a)...Ch. 20 - Prob. 20.91SPCh. 20 - Prob. 20.92SPCh. 20 - Prob. 20.93SPCh. 20 - Prob. 20.94SPCh. 20 - Prob. 20.95SPCh. 20 - Prob. 20.96SPCh. 20 - Prob. 20.97SPCh. 20 - Prob. 20.98SPCh. 20 - Prob. 20.99SPCh. 20 - Prob. 20.100SPCh. 20 - Prob. 20.101SPCh. 20 - Prob. 20.102SPCh. 20 - Prob. 20.103SPCh. 20 - Prob. 20.104SPCh. 20 - Prob. 20.105SPCh. 20 - For each of the following complexes, draw a...Ch. 20 - Prob. 20.107SPCh. 20 - Prob. 20.108SPCh. 20 - Prob. 20.109SPCh. 20 - Prob. 20.110SPCh. 20 - Prob. 20.111SPCh. 20 - Prob. 20.112SPCh. 20 - Prob. 20.113SPCh. 20 - Prob. 20.114CHPCh. 20 - Prob. 20.115CHPCh. 20 - Prob. 20.116CHPCh. 20 - Prob. 20.117CHPCh. 20 - Prob. 20.118CHPCh. 20 - Prob. 20.119CHPCh. 20 - Prob. 20.120CHPCh. 20 - Prob. 20.121CHPCh. 20 - Prob. 20.122CHPCh. 20 - Prob. 20.123CHPCh. 20 - Prob. 20.124CHPCh. 20 - Draw a crystal field energy-level diagram, and...Ch. 20 - Prob. 20.126CHPCh. 20 - Prob. 20.127CHPCh. 20 - Prob. 20.128CHPCh. 20 - Prob. 20.129CHPCh. 20 - Prob. 20.130CHPCh. 20 - Look at the colors of the isomeric complexes in...Ch. 20 - The amount of paramagnetism for a first-series...Ch. 20 - Prob. 20.133CHPCh. 20 - Prob. 20.134CHPCh. 20 - Prob. 20.135CHPCh. 20 - For each of the following, (i) give the systematic...Ch. 20 - Prob. 20.137CHPCh. 20 - Prob. 20.138CHPCh. 20 - Prob. 20.139MPCh. 20 - Formation constants for the ammonia and...Ch. 20 - Prob. 20.141MPCh. 20 - Prob. 20.143MPCh. 20 - An alternative to cyanide leaching of gold ores is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 10. Stereochemistry. Assign R/S stereochemistry for the chiral center indicated on the following compound. In order to recieve full credit, you MUST SHOW YOUR WORK! H₂N CI OH CI カー 11. () Stereochemistry. Draw all possible stereoisomers of the following compound. Assign R/S configurations for all stereoisomers and indicate the relationship between each as enantiomer, diastereomer, or meso. NH2 H HNH, -18arrow_forwardb) 8. Indicate whether the following carbocation rearrangements are likely to occur Please explain your rational using 10 words or less not likely to occur • The double bond is still in the Same position + Likely to oc occur WHY? -3 H3C Brave Chair Conformers. Draw the chair conformer of the following substituted cyclohexane. Peform a RING FLIP and indicate the most stable conformation and briefly explain why using 20 words or less. CI 2 -cobs ?? MUST INDICATE H -2 -2 Br EQ Cl OR AT Br H& most stable WHY? - 4arrow_forwardCH 12 Conformational Analysis. Draw all 6 conformers (one above each letter) of the compound below looking down the indicated bond. Write the letter of the conformer with the HIGHEST and LOWEST in energies on the lines provided. NOTE: Conformer A MUST be the specific conformer of the structure as drawn below -4 NOT HOH OH 3 Conformer A: Br OH A Samo Br H 04 Br H H3 CH₂ H anti stagere Br CH clipsed H Brott H IV H MISSING 2 -2 B C D E F X 6 Conformer with HIGHEST ENERGY: 13. (1 structure LOWEST ENERGY: Nomenclature. a) Give the systematic (IUPAC) name structure. b) Draw the corresponding to this name. HINT: Do not forget to indicate stereochemistry when applicable. a) ८८ 2 "Br {t༐B,gt)-bemn€-nehpརི་ཚ༐lnoa Parent name (noname) 4 Bromo Sub = 2-methylethyl-4 Bromo nonane b) (3R,4S)-3-chloro-4-ethyl-2,7-dimethyloctane # -2 -2arrow_forward
- in the scope of the SCH4U course! please show all steps as im still learning how to format my answers in the format given, thank you!arrow_forwardhelp me solve this HWarrow_forwardMolecules of the form AH2 can exist in two potential geometries: linear or bent. Construct molecular orbital diagrams for linear and bent CH2. Identify the relevant point group, include all of the appropriate symmetry labels and pictures, and fill in the electrons. Which geometry would you predict to be more stable, and why? (Please draw out the diagram and explain)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry; Author: Crash Chemistry Academy;https://www.youtube.com/watch?v=apuWi_Fbtys;License: Standard YouTube License, CC-BY