
Concept explainers
(a)
Interpretation:
The preparation of the below given compound using directed aldol reactions has to be given.
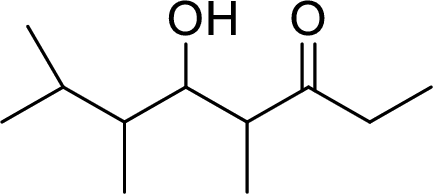
Concept Introduction:
In directed aldol reactions, the enolate anion is prepared with one carbonyl compound by LDA (Lithium diisopropylamide). The other carbonyl compound acts as an electrophile. Both of the carbonyl compounds have α-hydrogens, but only one enolate is prepared using LDA. When unsymmetrical ketone is used, it forms less substituted enolate.
LDA (Lithium diisopropylamide) is a strong base. It converts
(b)
Interpretation:
The preparation of the below given compound using directed aldol reactions has to be given.
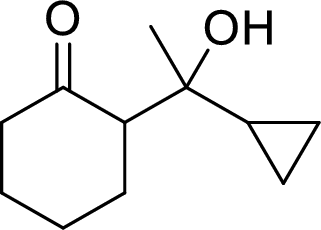
Concept Introduction:
In directed aldol reactions, the enolate anion is prepared with one carbonyl compound by LDA (Lithium diisopropylamide). The other carbonyl compound acts as an electrophile. Both of the carbonyl compounds have α-hydrogens, but only one enolate is prepared using LDA. When unsymmetrical ketone is used, it forms less substituted enolate.
LDA (Lithium diisopropylamide) is a strong base. It converts aldehydes, ketones and esters into their enolate anions.
Trending nowThis is a popular solution!

Chapter 19 Solutions
Organic Chemistry
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
