
(a)
Interpretation:
Reagents and experimental conditions by which an oxanamide can synthesized from butanal has to be shown.
Concept introduction:
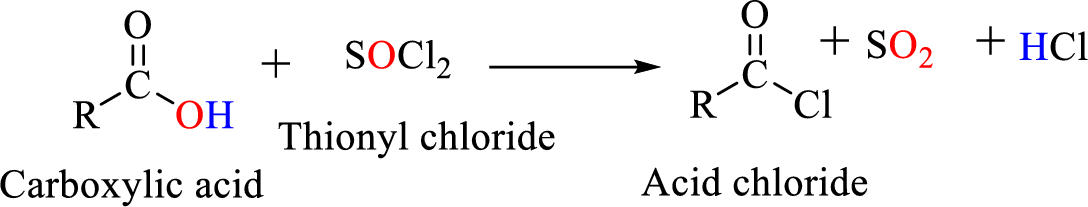
Acid chlorides are most often prepared by treating a
Alkyl or aryl magnesium halides (RMgX) are known as Grignard reagent. The Grignard reaction is an organometallic
Reaction of Grignard reagent with a formic ester followed by hydrolysis in aqueous acid yields secondary alcohol. Tertiary alcohol can be prepared by the reaction of Grignard reagent with ester other than formate.
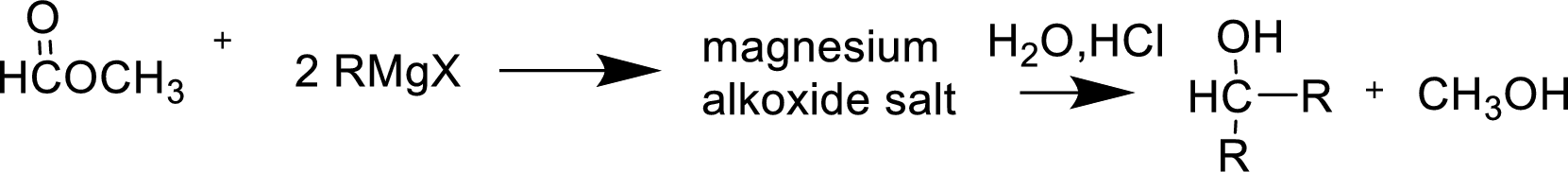
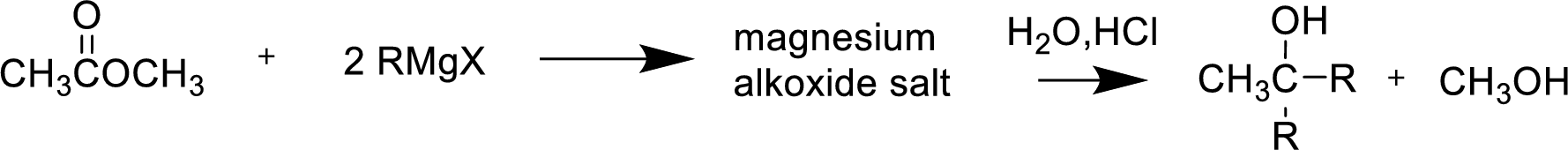
(b)
Interpretation:
The number of chiral centers and stereoisomers possible for the given compound has to be shown.
Concept introduction:
Chiral centre:
A chiral centre is defined as the tetrahedral carbon atom in an organic molecule that is connected to four non-identical groups/substituents. It is sometimes known as a stereo genic centre.
Stereoisomers: Two compounds with same molecular formula but different in their orientation are considered as isomers.
Number of possible stereoisomers for a compound can be determined as,
Trending nowThis is a popular solution!

Chapter 19 Solutions
Organic Chemistry
- I would like my graphs checked please. Do they look right? Do I have iodine and persulfate on the right axis ?arrow_forwardReaction Fill-ins Part 2! Predict the product(s) OR starting material of the following reactions. Remember, Hydride shifts are possible if/when a more stable carbocation can exist (depending on reaction mechanism)! Put your answers in the indicated boxes d. d. ง HCIarrow_forwardA cylinder contains 12 L of water vapour at 150˚C and 5 atm. The temperature of the water vapour is raised to 175˚C, and the volume of the cylinder is reduced to 8.5 L. What is the final pressure of the gas in atmospheres? assume that the gas is idealarrow_forward
- On the next page is an LC separation of the parabens found in baby wash. Parabens are suspected in a link to breast cancer therefore an accurate way to quantitate them is desired. a. In the chromatogram, estimate k' for ethyl paraben. Clearly indicate what values you used for all the terms in your calculation. b. Is this a "good" value for a capacity factor? Explain. c. What is the resolution between n-Propyl paraben and n-Butyl paraben? Again, indicate clearly what values you used in your calculation. MAU | Methyl paraben 40 20 0 -2 Ethyl paraben n-Propyl paraben n-Butyl paraben App ID 22925 6 8 minarrow_forwardd. In Figure 4, each stationary phase shows some negative correlation between plate count and retention factor. In other words, as k' increases, N decreases. Explain this relationship between k' and N. Plate Count (N) 4000 3500 2500 2000 1500 1000 Figure 4. Column efficiency (N) vs retention factor (k') for 22 nonionizable solutes on FMS (red), PGC (black), and COZ (green). 3000 Eluent compositions (acetonitrile/water, A/W) were adjusted to obtain k' less than 15, which was achieved for most solutes as follows: FMS (30/70 A/W), PGC (60/40), COZ (80/20). Slightly different compositions were used for the most highly retained solutes. All columns were 50 mm × 4.6 mm id and packed with 5 um particles, except for COZ, which was packed with 3 um particles. All other chromatographic conditions were constant: column length 5 cm, column j.§. 4.6 mm, flow rate 2 mL/min, column temperature 40 °C, and injection volume 0.5 μL Log(k'x/K'ethylbenzene) FMS 1.5 1.0 0.5 0.0 ཐྭ ཋ ཤྩ བྷྲ ; 500 0 5 10…arrow_forwardf. Predict how the van Deemter curve in Figure 7 would change if the temperature were raised from 40 °C to 55 °C. Figure 7. van Desmter curves in reduced coordinates for four nitroalkane homologues (nitropropane, black; nitrobutane, red; nitropentane, blue; and nitrohexane, green) separated on the FMS phase. Chromatographic conditions: column dimensions 50 mm × 4.6 mm id, eluent 30/70 ACN/water, flow rates 0.2-5.0 mL/min, injection volume 0.5 and column temperature 40 °C. No corrections to the plate heights have been made to account for extracolumn dispersion. Reduced Plate Height (h) ° 20 40 60 Reduced Velocity (v) 8. (2) A water sample is analyzed for traces of benzene using headspace analysis. The sample and standard are spiked with a fixed amount of toluene as an internal standard. The following data are obtained: Ppb benzene Peak area benzene Peak area toluene 10.0 252 376 Sample 533 368 What is the concentration of benzene in the sample?arrow_forward
- Liquid chromatography has been used to track the concentration of remdesivir (a broad-spectrum antiviral drug, structure shown at right) in COVID patients undergoing experimental treatments. Intensity The authors provide the following details regarding standard solutions preparation: HN CN HO OH NH2 Remdesivir (RDV) stock solution (5000 µg/mL) was prepared by dissolving RDV drug powder using the mixture of DMSO: MeOH (30:70 v/v). The RDV working standard solutions for calibration and quality controls were prepared using methanol in concentrations of 100, 10, 1, 0.1, 0.01 µg/mL. 1, 2.5, 5, 7.5, 10, 25, 50, 75, 100, 250, 500, 1000, and 5000 ng/mL sample solutions were prepared freshly by spiking calibration standard solutions into the blank human plasma samples for method calibration. a) What type of calibration method is being described? Why do you think the authors chose this method as opposed to another? b) Based on the details provided in part a, describe an appropriate method blank…arrow_forwardRecent advancements in liquid chromatography include the development of ultrahigh pressure liquid chromatography (UHPLC) and an increased use of capillary columns that had previously only been used with gas chromatography. Both of these advances have made the development of portable LC systems possible. For example, Axcend Corp. makes a portable system that uses a capillary column with an internal diameter of 150-μm-that is packed with 1.7-um stationary phase particles. In contrast, a traditional LC column has a 4.6 mm internal diameter and utilizes 5-um stationary phase particles. a) Explain one advantage that is afforded by the use of a capillary column in liquid chromatographic separation. Explain one disadvantage of capillary columns. b) Explain how the use of smaller stationary phase particles can improve the resolution of a separation. Include any relevant equations that support your explanation. c) A scientist at Rowan University is using the Axcend LC to conduct analyses of F…arrow_forwardThis paper describes the use of fullerene molecules, also known as buckyballs, as a stationary phase for liquid chromatography. The performance of the fullerene-modified stationary phase (FMS) is compared to that of a more common C18 stationary phase and to two other carbon-based stationary phases, PGC and COZ. A. 10A OM B. - Figure 1. Idealized drawing of the cross-section of a pore inside a silica particle, showing the relative densities of aminopropylsilyl (red/green) and fullerene (blue) groups: (A) full cross- section; (B) detailed view of covalent bonding of fullerene to the silica surface. Surface densities of silyl and fullerene groups were inferred from elemental composition results obtained at each stage of the synthesis (see Table 1). Absorbance (mAU, 220 nm) 700 600 500 400 300 200 100 a. Define selectivity, a, with words and an equation. b. Explain how the choice of stationary phase affects selectivity. c. Calculate the resolution of the nitrobenzene and toluene peaks in…arrow_forward
- Normalized Intensity (a. u.) 0.5 1.0 A 3D-printed GC column (shown below) was created for use with "micro" gas chromatography applications. To prove its utility, it was used to separate a mixture of alkanes (C9-C18, C22, C24). For the separation shown below, the column temperature was ramped from 40 °C to 250 °C at a rate of 30 °C per minute. (a) 9 10 = 1 mm 12 13 15 22 0.0 0 100 200 300 400 Time (sec) a) What detector would you use for this analysis? Justify your selection. b) Explain how the chromatogram would change if the separation was run isothermally. c) Explain how the chromatogram would change if the temperature ramp were increased to 50 °C per minute.arrow_forwardDevise a synthesis of each compound from the indicated starting material. You may also use any organic compounds with one or two carbons and any needed inorganic reagents. a. Brarrow_forwardPlease help me with #2b & #3 using the data.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





