
Concept explainers
(a)
Interpretation:
Whether NAD+ is involved in (1) glycerol
Concept introduction:
Triacylglycerol mobilization is an ongoing process in which triacylglycerols that are stored in the adipose tissue are hydrolyzed. Fatty acids and glycerol are the products of triacylglycerol mobilization. The products are released into the bloodstream.
After entering the bloodstream, the glycerol travels to the kidneys or liver. The first stage of glycerol metabolism occurs in the liver or kidney. The first stage of glycerol metabolism is a two-step process. After the first stage, the remaining stages of glycerol metabolism are the same as glucose pathways. The overall equation for glycerol metabolism is as follows:
Fatty acids are molecules that are long hydrocarbon chain of
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as a β-oxidation pathway. Acetyl CoA, FADH2, and NADH are produced in this pathway.
Nicotinamide adenine dinucleotide is associated with the
(a)
Answer to Problem 14.47EP
NAD+ is involved in (3) both glycerol metabolism and fatty acid metabolism.
Explanation of Solution
The first stage of glycerol metabolism is a two-step process. In step 1, glycerol-3-phosphate is formed as the intermediate compound that further reacts to form dihydroxyacetone phosphate in step 2. The reaction for the conversion of glycerol is as follows:
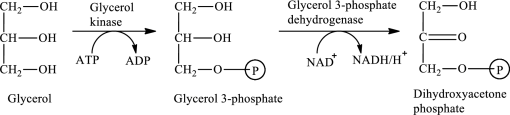
Here, represents
represents
In step 2 of glycerol metabolism, NAD+ oxidized glycerol-3-phosphate to dihydroxyacetone phosphate. Therefore, NAD+ is involved in glycerol metabolism.
The reaction in step 3 of a turn of the β-oxidation pathway is a dehydrogenation reaction in which two hydrogen atoms are removed from L-β-hydroxyacyl CoA. In this reaction, the β-hydroxy group is converted to a β-keto group. NAD+ is used as an oxidizing agent. This reaction is catalyzed by β-hydroxyacyl CoA dehydrogenase enzyme. The reaction for step 3 is as follows:
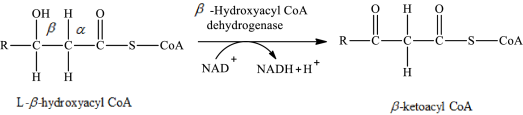
Therefore, NAD+ is involved in (3) both glycerol metabolism and fatty acid metabolism.
(b)
Interpretation:
Whether ADP is involved in (1) glycerol metabolism to dihydroxyacetone phosphate, (2) fatty acid metabolism to acetyl CoA, or (3) both glycerol metabolism and fatty acid metabolism has to be determined.
Concept introduction:
Triacylglycerol mobilization is an ongoing process in which triacylglycerols that are stored in the adipose tissue are hydrolyzed. Fatty acids and glycerol are the products of triacylglycerol mobilization. The products are released into the bloodstream.
After entering the bloodstream, the glycerol travels to the kidneys or liver. The first stage of glycerol metabolism occurs in the liver or kidney. The first stage of glycerol metabolism is a two-step process. After the first stage, the remaining stages of glycerol metabolism are the same as glucose pathways. The overall equation for glycerol metabolism is as follows:
Fatty acids are molecules that are long hydrocarbon chain of carboxylic acid. They are building blocks of fat in humans and animals.
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as a β-oxidation pathway. Acetyl CoA, FADH2, and NADH are produced in this pathway.
Adenosine diphosphate (ADP) provides energy to carry out the metabolic processes in the living cells.
(b)
Answer to Problem 14.47EP
ADP is involved in (1) glycerol metabolism to dihydroxyacetone phosphate.
Explanation of Solution
The first stage of glycerol metabolism is a two-step process. In step 1, glycerol-3-phosphate is formed as the intermediate compound that further reacts to form dihydroxyacetone phosphate in step 2. The reaction for the conversion of glycerol is as follows:
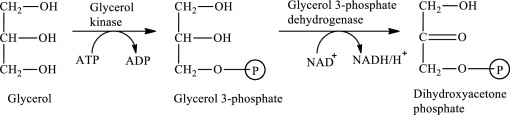
Here, represents
represents
In step 1 of glycerol metabolism, ATP is converted to ADP. Therefore, ADP is involved in glycerol metabolism.
(c)
Interpretation:
Whether kinase is involved in (1) glycerol metabolism to dihydroxyacetone phosphate, (2) fatty acid metabolism to acetyl CoA, or (3) both glycerol metabolism and fatty acid metabolism has to be determined.
Concept introduction:
Triacylglycerol mobilization is an ongoing process in which triacylglycerols that are stored in the adipose tissue are hydrolyzed. Fatty acids and glycerol are the products of triacylglycerol mobilization. The products are released into the bloodstream.
After entering the bloodstream, the glycerol travels to the kidneys or liver. The first stage of glycerol metabolism occurs in the liver or kidney. The first stage of glycerol metabolism is a two-step process. After the first stage, the remaining stages of glycerol metabolism are the same as glucose pathways. The overall equation for glycerol metabolism is as follows:
Fatty acids are molecules that are long hydrocarbon chain of carboxylic acid. They are building blocks of fat in humans and animals.
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as a β-oxidation pathway. Acetyl CoA, FADH2, and NADH are produced in this pathway.
The transfer of a phosphoryl group
(c)
Answer to Problem 14.47EP
Kinase is involved in (1) glycerol metabolism to dihydroxyacetone phosphate.
Explanation of Solution
The first stage of glycerol metabolism is a two-step process. In step 1, glycerol-3-phosphate is formed as the intermediate compound that further reacts to form dihydroxyacetone phosphate in step 2. The reaction for the conversion of glycerol is as follows:
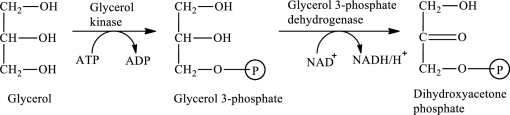
Here, represents
represents
In step 1 of glycerol metabolism, glycerol kinase enzyme catalyzed the conversion of glycerol to glycerol-3-phosphate. Therefore, the kinase is involved in glycerol metabolism.
(d)
Interpretation:
Whether ketoacyl CoA is involved in (1) glycerol metabolism to dihydroxyacetone phosphate, (2) fatty acid metabolism to acetyl CoA, or (3) both glycerol metabolism and fatty acid metabolism has to be determined.
Concept introduction:
Triacylglycerol mobilization is an ongoing process in which triacylglycerols that are stored in the adipose tissue are hydrolyzed. Fatty acids and glycerol are the products of triacylglycerol mobilization. The products are released into the bloodstream.
After entering the bloodstream, the glycerol travels to the kidneys or liver. The first stage of glycerol metabolism occurs in the liver or kidney. The first stage of glycerol metabolism is a two-step process. After the first stage, the remaining stages of glycerol metabolism are the same as glucose pathways. The overall equation for glycerol metabolism is as follows:
Fatty acids are molecules that are long hydrocarbon chain of carboxylic acid. They are building blocks of fat in humans and animals.
The fatty acids are broken down to provide energy. The breakdown of fatty acids is a three parts process. In the first part, the fatty acid is activated. In the second part, the transportation of fatty acid into the mitochondrial matrix is facilitated by a shuttle mechanism. In the third part, the fatty acid is readily oxidized, cycling through a series of four reactions. In these series of reactions, acyl CoA is degraded to acetyl CoA. This pathway is termed as a β-oxidation pathway. Acetyl CoA, FADH2, and NADH are produced in this pathway.
Nicotinamide adenine dinucleotide is associated with the redox reactions in metabolism. Its reduced form is NADH and oxidized form is NAD+.
(d)
Answer to Problem 14.47EP
Ketoacyl CoA is involved in (2) fatty acid metabolism to acetyl CoA.
Explanation of Solution
The reaction in step 3 of a turn of the β-oxidation pathway is a dehydrogenation reaction in which two hydrogen atoms are removed from L-β-hydroxyacyl CoA. In this reaction, the β-hydroxy group is converted to a β-keto group. NAD+ is used as an oxidizing agent. This reaction is catalyzed by β-hydroxyacyl CoA dehydrogenase enzyme. The reaction for step 3 is as follows:
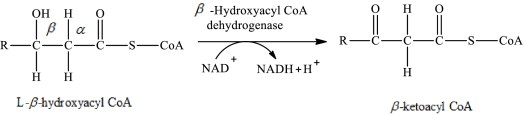
Therefore, ketoacyl CoA is involved in (2) fatty acid metabolism to acetyl CoA.
Want to see more full solutions like this?
Chapter 14 Solutions
Organic And Biological Chemistry
- : Draw the structure of valylasparagine, a dipeptide made from valine and asparagine, as it would appear at physiological pH. Click and drag to start drawing a structure. P Darrow_forwardDraw the Haworth projection of α-L-mannose. You will find helpful information in the ALEKS Data resource. Click and drag to start drawing a structure. : ཊི Х Darrow_forwardDraw the structure of serine at pH 6.8. Click and drag to start drawing a structure. : d كarrow_forward
- Take a look at this molecule, and then answer the questions in the table below it. CH2OH H H H OH OH OH CH2OH H H H H OH H H OH H OH Is this a reducing sugar? yes α β ロ→ロ no ☑ yes Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. O no 0+0 If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. ☐arrow_forwardAnswer the questions in the table below about this molecule: H₂N-CH₂ -C—NH–CH–C—NH–CH—COO- CH3 CH CH3 What kind of molecule is this? 0= CH2 C If you said the molecule is a peptide, write a description of it using 3-letter codes separated ☐ by dashes. polysaccharide peptide amino acid phospolipid none of the above Хarrow_forwardDraw a Haworth projection of a common cyclic form of this monosaccharide: CH₂OH C=O HO H H -OH H OH CH₂OH Click and drag to start drawing a structure. : ☐ Х S '☐arrow_forward
- Nucleophilic Aromatic Substitution 22.30 Predict all possible products formed from the following nucleophilic substitution reactions. (a) (b) 9 1. NaOH 2. HCI, H₂O CI NH₁(!) +NaNH, -33°C 1. NaOH 2. HCl, H₂Oarrow_forwardSyntheses 22.35 Show how to convert toluene to these compounds. (a) -CH,Br (b) Br- -CH3 22.36 Show how to prepare each compound from 1-phenyl-1-propanone. 1-Phenyl-1-propanone ہتی. Br. (b) Br (racemic) 22.37 Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid. 22.38 Show reagents and conditions to bring about the following conversions. (a) 9 NH2 8 CO₂H NH2 CO₂Et (d) NO2 NH2 S NH₂ NO2 CHS CHarrow_forwardive the major organic product(s) of each of the following reactions or sequences of reactions. Show all rant stereochemistry. [10 only] A. B. NaN3 1. LiAlH4, ether Br 2. H₂O CH3 HNO3 H₂/Pt H₂SO ethanol C. 0 0 CH3CC1 NaOH NHCCH AICI H₂O . NH₂ CH3CH2 N CH2CH3 + HCI CH₂CH 3 1. LIAIH, THE 2. H₂Oarrow_forward
- Calculate the stoichiometric amount of CaCl2 needed to convert all of the CuSO4 into CuCl2.arrow_forwardH CH تنی Cl 1. NaCN, DMF 2. LIAIH4, ether H₂O pyridine N NH₂ 5 CH H 1 HNO, H₂SO 2. Nal NH2 Br Br HNO₂ CuCl H₂SO HCI CH3 H3C NN HSO KCN CuCN 1. HNO₂, H₂SO O₂N NH2 2. OH ཀ་ལས། །ས་ཅན་ :i་དེ་མ་མ་སེ་ NH₂ CH3 1. HNO₂, H₂SO4 2. H3PO₂ 1 HNO2, H2SO4 2. Nalarrow_forwardive the major organic product(s) of each of the following reactions or sequences of reactions. Show all rant stereochemistry. [10 only] A. B. NaN3 1. LiAlH4, ether Br 2. H₂O CH3 HNO3 H₂/Pt H₂SO ethanol C. 0 0 CH3CC1 NaOH NHCCH AICI H₂O . NH₂ CH3CH2 N CH2CH3 + HCI CH₂CH 3 1. LIAIH, THE 2. H₂Oarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



